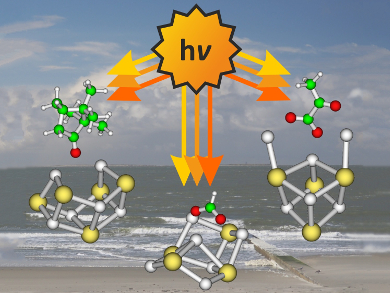
Marine aerosols play an essential role in many atmospheric processes. Their major components are salts, water, and organic compounds. The aging of such sea-salt aerosols in sunlight is a complex process.
Martin Beyer, Milan Ončák, and colleagues, Innsbruck University, Austria, have used sodium iodide clusters doped with hydrocarbons (camphor, formic acid, and pyruvic acid) as models for marine aerosols. The team produced the clusters via electrospray ionization, “stored” them in a mass spectrometer, and irradiated them with both ultraviolet (UV) and infrared (IR) laser light to study their spectroscopic properties and photoinduced decomposition. Quantum chemical calculations were performed to improve the understanding of the resulting spectra and processes.
The researchers found that the salt environment efficiently quenches photoexcitation: When the organic molecule is excited, it transfers its energy to the salt cluster, which then dissociates. The hydrocarbons, thus, stay intact. Their photochemical lifetime is increased in sea-salt aerosols compared with molecules in the gas-phase. Only in the case of pyruvate, traces of the elusive CO2·–-radical anion were observed, produced by photolysis of the pyruvate C–C bond.
- Photodissociation of Sodium Iodide Clusters Doped with Small Hydrocarbons,
Nina K. Bersenkowitsch, Milan Ončák, Jakob Heller, Christian van der Linde, Martin K. Beyer,
Chem. Eur. J. 2018.
https://doi.org/10.1002/chem.201803017