Chemical ligation strategies, i.e., chemoselective couplings of peptides, are commonly used for the modification of proteins or the inclusion of unnatural amino acids. Native chemical ligation has been the leading methodology for synthetic access to complex proteins. The method involves an attack by the thiol group of an N-terminal cysteine residue of one peptide on a C-terminal thioester of a second peptide. However, the lack of robust tools to generate the requisite thioester peptides (pictured right) on demand limits the method’s utility.
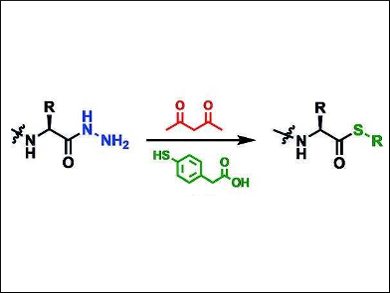
Philip E. Dawson, The Scripps Research Institute, La Jolla, CA, USA, and colleagues have developed a method for the synthesis of thioester peptides that relies on a mild and stoichiometric activation of peptide hydrazides (pictured). The peptide hydrazides are converted to aryl thioesters through treatment with acetylacetone (acac) in an acidic buffer containing an aryl thiol. The reaction proceeds via a pyrazole intermediate.
This method allows for one-pot sequential ligations and is compatible with the thiazolidine protection of cysteines. Intermediate purifications are minimized. These results also demonstrate the usefulness of acylpyrazoles as acylation agents in organic synthesis.
- Leveraging the Knorr Pyrazole Synthesis for the Facile Generation of Thioester Surrogates for use in Native Chemical Ligation,
Dillon T. Flood, Jordi C. J. Hintzen, Michael J. Bird, Philip A. Cistrone, Jason S. Chen, Philip E. Dawson,
Angew. Chem. Int. Ed. 2018.
https://doi.org/10.1002/anie.201805191