Adenosine triphosphate (ATP) is the energy source for most cellular processes. ATP is used by kinase enzymes, which transfer a phosphate group from ATP on to proteins. This phosphorylation process plays a key role in cellular signaling and controlling enzyme activity. Misregulation of this process is the primary cause of many types of cancer. To gain a better understanding of these cellular processes, new tools are needed for visualizing ATP in living cells. It is currently very difficult to detect ATP selectively, due to the large number of structurally similar anions present in cells (e.g., ADP, GTP, AMP).
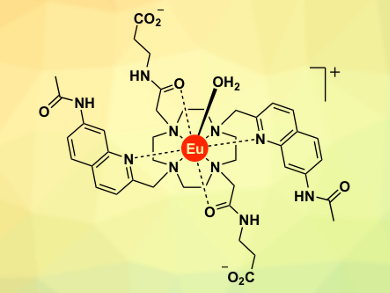
Stephen Butler, Loughborough University, UK, and colleagues have developed a series of europium(III) complexes (example pictured) designed to bind reversibly to ATP and provide a distinctive, long-lived luminescent signal. By incorporating hydrogen-bonding units into the “binding arms” of the Eu(III) complex and changing the nature of the surrounding ligand, it was possible to modulate the strength of anion binding and the luminescence response.
This allowed ATP to be distinguished from other common phosphate anions, including ADP, GTP, and AMP, in an aqueous solution containing biologically relevant concentrations of Mg2+ ions and protein. This enabled the researchers to continuously monitor the enzymatic conversion of ATP to ADP using luminescence spectroscopy.
The team showed that the Eu(III) complexes accumulate preferentially within the mitochondria of living cells. Using fluorescence microscopy, they were able to visualize elevated ATP levels within the mitochondria after treating cells with an inhibitor of kinase activity. It was also possible to monitor depleted ATP levels, following the treatment of cells with potassium cyanide, an inhibitor of ATP synthesis. The synthesized Eu(III) probes could provide a versatile imaging tool for studying cell metabolism and a range of ATP-requiring processes in real-time.
- Cationic Europium Complexes for Visualizing Fluctuations in Mitochondrial ATP Levels in Living Cells,
Romain Mailhot, Thomas Traviss-Pollard, Robert Pal, Stephen J. Butler,
Chem. Eur. J. 2018, 24, 10745–10755.
https://doi.org/10.1002/chem.201801008