Phosphines are among the most important ligands in transition-metal chemistry and are indispensable for homogeneously catalyzed reactions such as cross-couplings, hydrofunctionalizations, and hydrogenations. Advances in this field are often based on new phosphine ligands that tune the steric and electronic properties of the active metal.
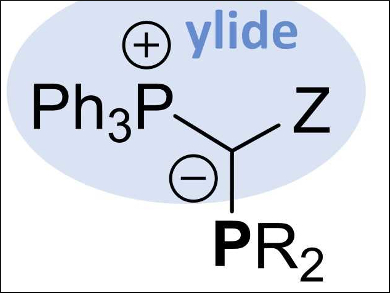
Viktoria H. Gessner, Universities of Bochum and Würzburg, both Germany, and colleagues have developed a class of ylide-substituted phosphines, named YPhos (pictured). The introduction of ylide substituents in phosphine ligands is done via three alternative routes, all starting from commercially available starting materials: 1) a double deprotonation of phosphonium salts and a reaction with a halophosphine, 2) a reaction of the corresponding ylide with a dialkylhalophosphine, or 3) deprotonation of an α‐phosphino substituted phosphonium salt.
The steric and electronic properties of the ligand can be tuned by varying the substituents. The incorporated ylide moiety makes them electron-rich, and thus, they have donor strengths greater than those of commonly available phosphines and carbenes.
The team explored the catalytic potential of the YPhos ligands in the gold-catalyzed hydroamination of alkynes with amines. The tested systems achieved excellent performance at low catalyst loadings for a range of substrates under mild conditions. The researchers believe the steric bulk of YPhos ligands could stabilize low-coordinate transition-metal compounds for further catalytic applications.
- Ylide-Functionalized Phosphines: Strong Donor Ligands for Homogenous Catalysis,
Thorsten Scherpf, Christopher Schwarz, Lennart T. Scharf, Jana-Alina Zur, Andreas Helbig, Viktoria H. Gessner,
Angew. Chem. Int. Ed. 2018.
https://doi.org/10.1002/anie.201805372


![Unique Features of the Dinuclear Zirconocene Complex [(Cp₂Zr)₂(µ-Me)(µ-C₂Ph)]](https://www.chemistryviews.org/wp-content/uploads/2025/03/202503_Dinuclear-Zirconocene-Complex-125x94.png)