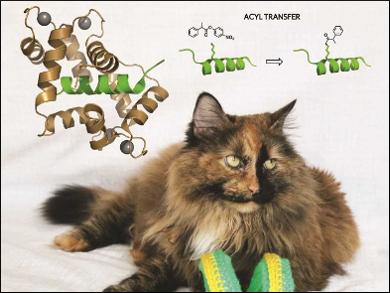
The introduction of post-translational modifications into proteins is an extremely complex but very important task. Ivan Korendovych, Olga Makhlynets, and colleagues, Syracuse University, NY, USA, have designed an enzyme that can do just that—the enzyme is an efficient and site-selective catalyst for the post‐translational acylation of lysines in peptides.
Calmodulin (CaM) is one of the most abundant eukaryotic proteins. It modulates the activities of a variety of important enzymes and ion channels. Calmodulin recognizes a pattern of charged and hydrophobic residues rather than a specific sequence to bind highly diverse targets with high affinity.
While calmodulin itself has no known catalytic activity, the incorporation of a single mutation converts it into an efficient esterase, CaM M144H, that selectively transfers an acyl group from a small molecule onto calmodulin-binding partners. CaM M144H is a variant of a modified enzyme the researchers had previously developed and termed AlleyCatE [1].
The resulting post-translational modifications can be detected by mass spectrometry. While further studies will be needed to test the full limits of its use, CaM M144H has the potential to become a valuable chemical biology tool to identify the calmodulin-binding domains in complex biological systems.
- A Designed Enzyme Promotes Selective Post-translational Acylation,
Pallavi M. Gosavi, Megha Jayachandran, Joel J. L. Rempillo, Oleksii Zozulia, Olga V. Makhlynets, Ivan V. Korendovych,
ChemBioChem 2018.
https://doi.org/10.1002/cbic.201800196
Reference
- New Tricks for Old Proteins: Single Mutations in a Nonenzymatic Protein Give Rise to Various Enzymatic Activities,
Yurii S. Moroz, Tiffany T. Dunston, Olga V. Makhlynets, Olesia V. Moroz, Yibing Wu, Jennifer H. Yoon, Alissa B. Olsen, Jaclyn M. McLaughlin, Korrie L. Mack, Pallavi M. Gosavi, Nico A. J. van Nuland, Ivan V. Korendovych,
J. Am. Chem. Soc. 2015, 137, 14905–14911.
https://doi.org/10.1021/jacs.5b07812