Malaria and tuberculosis are health problems which are especially common in developing countries A major roadblock to the eradication of such diseases is their resistance to currently available drugs. Repurposing existing drugs in the form of new derivatives is one approach to solve this problem. An example of this is the organo-iron derivative of the antimalarial drug chloroquine, ferroquine, which is now in the clinical trial phase.
Peter Sadler, Prinessa Chellan, University of Warwick, UK, and colleagues have investigated if the organometallic derivatization of sulfadoxine, a drug used to treat malaria and other microbial diseases, could enhance its activity. The researchers screened the effectiveness of arene-ruthenium(II), cyclopentadienyl-iridium(III), and cyclopentadienyl-rhodium(III) derivatives of sulfadoxine against various microbes, including Plasmodium falciparum, the malaria strain that infects humans, and Mycobacterium tuberculosis.
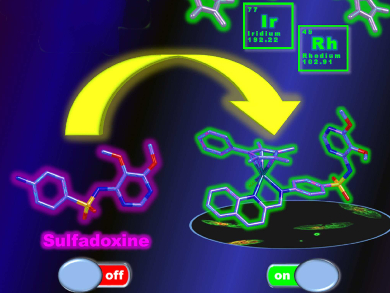
Sulfadoxine itself has little activity against these pathogens. However, the organo-Rh and organo-Ir complexes were good inhibitors, effectively “switching on” the activity of sulfadoxine (pictured). Notably, some metallo-sulfadoxines were active against late-stage gametocytes (LSG) of the malaria parasites, whereas the drugs sulfadoxine, pyrimethamine, and chloroquine were not. This is a significant finding because LSGs cause the spread of the disease by transfer from a human host to female Anopheles mosquitos, which can then infect another person.
- Organometallic Conjugates of the Drug Sulfadoxine for Combatting Antimicrobial Resistance,
Prinessa Chellan, Vicky M. Avery, Sandra Duffy, James A. Triccas, Gayathri Nagalingam, Christina Tam, Luisa W. Cheng, Jenny Liu, Kirkwood M. Land, Guy J. Clarkson, Isolda Romero-Canelón, Peter J. Sadler,
Chem. Eur. J. 2018.
https://doi.org/10.1002/chem.201801090