Metal-catalyzed reactions could be made “greener” and more sustainable by replacing expensive noble metals such as iridium, ruthenium, palladium, and rhodium with earth-abundant, environmentally friendly, first-row transition metals such as iron, cobalt, nickel, and manganese.
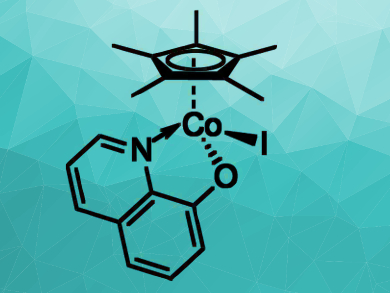
Basker Sundararaju, Indian Institute of Technology, Kanpur, and colleagues have developed a well-defined, air- and moisture-stable Cp*Co(III) complex (pictured) for the efficient dehydrogenation of secondary alcohols into carbonyl compounds and for dehydrative ether synthesis under mild conditions. The dehydrogenations were performed using 1 mol% of the Cp*Co(III) catalyst at 80 °C for 24 h and gave the desired products in moderate to good yields.
The reaction tolerates a wide range of substrates, including both aliphatic and aromatic secondary alcohols. According to the researchers, the reaction proceeds via a hydrogen-atom transfer process mediated by the high-valent cobalt species. The developed Cp*Co(III)-based complex could also be useful for other reactions that involve a hydrogen-borrowing strategy.
- Cp*Co(III)-catalyzed Efficient Dehydrogenation of Secondary Alcohols,
Manoj Kumar Gangwar, Pardeep Dahiya, Balakumar Emayavaramban, Basker Sundararaju,
Chem. Asian J. 2018.
https://doi.org/10.1002/asia.201800697