Condensed-phase spectroscopy is a powerful tool to study solvated species. However, it is crucial to account for water solvent effects to correctly interpret the resulting spectra, as water is capable of forming extensive H‐bonding networks, making it difficult to justify how many water molecules should be included, and H‐bonding networks in bulk water are known to show short time dynamics. so currently, there is much debate on how many water molecules need to be considered to model the explicit hydrogen-bonding interactions between solute and water molecules, as well as the bulk water environment.
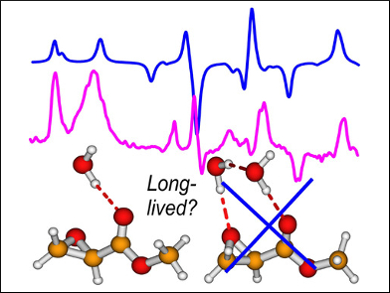
Yunjie Xu, University of Alberta, Canada, and colleagues have proposed a new approach—called the “clusters-in-a-liquid” model—which is based on chiroptical features induced in the solvent molecules. According to this method, small hydration clusters with just one or two water molecules attached to the solute are the long-lived species that make the dominant contributions to the observed solution spectra.
Infrared (IR), vibrational circular dichroism (VCD), Raman, and Raman optical activity (ROA) spectroscopic studies of methyl glycidate in water show excellent agreement between experiment and this proposed theory for all four types of spectra. The study provides insights into the different roles water molecules play in solution and points to a new efficient strategy for modeling water solvent effects.
- IR, Raman, and Vibrational Optical Activity Spectra of Methyl Glycidate in Chloroform and Water: The Clusters-in-a-liquid Solvation Model,
Angelo Shehan Perera, Joseph Cheramy, Christian Merten, Javix Thomas, Yunjie Xu,
ChemPhysChem 2018.
https://doi.org/10.1002/cphc.201800309