K. C. Nicolaou, Rice University, Houston, TX, USA, and colleagues have developed a total synthesis of a rare natural product isolated from a sea creature found along the coast of the Fijian island Namenalala more than twenty years ago. Tests on the compound show that it is a cell killer—causing cytotoxicity in leukemia cells in the laboratory—and also shows antimicrobial activity against a variety of bacteria.
A Novel Enediyne
Nature abounds with chemicals that are physiologically active. They often emerge from the most obscure organisms in remote locations, although others come from the bark of common trees and the microbes that grow in soil. It is estimated that around 40 % of prescription drugs have a natural product origin. The search for new structures with therapeutic potential is ongoing and new sources are constantly being probed.
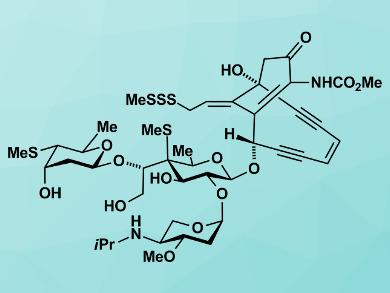
A rare natural product that goes by the name of namenamicin, an enediyne (pictured), was first detected and isolated in 1996 in an orange-colored sea squirt, Polysyncraton lithostrotum, by scientists at the University of Utah, Salt Lake City, USA and the pharmaceutical company Wyeth Ayerst Research Pearl River, New York, USA [1]. This sea squirt, or ascidian, was first collected off the Namenalalan coast.
Naming the Target
Initial research into namenamicin suggested that it was being produced as a metabolite by the micromonospora bacterium species that live a symbiotic life within the sea squirt. Haiyin He of Wyeth-Ayerst identified this source. Of course, it is present only in tiny quantities in the organism and its scarcity makes it difficult to obtain and carry out research on. As such, it is a target for the keen-eyed synthetic chemists who hope to be able to make usable amounts of the natural product, which is where one of the most prominent of those synthetic chemists, Nicolaou, and his colleagues stepped up.
The team points out that it is not only the puzzle of the structure with its numerous stereocenters, including an ambiguous one, that intrigues them but its possible role as an anticancer and antimicrobial agent. They write that namenamicin with its potent cytotoxic properties may prove useful as a lead compound payload for highly targeted antibody-delivered drug conjugates (ADCs). “Its scarcity, coupled with the uncertainty of its full absolute configuration, elevates it to an attractive synthetic target,” the team says.
Synthetic Approach to Cancer Therapy
The researchers have performed the total synthesis of the two C7′-epimers of namenamicin. This synthesis allowed them to assign the complete structure of the compound. “The absolute configuration of the rest of the molecule was assigned by analogy to the related marine natural product shishijimicin A,” the team reports.
The work opens the way to further chemical and biological studies. The primary aim would be the development of ADCs directed toward targeted cancer therapies. It has already been shown that the compound can treat P388 leukemia in mice and increase lifespan by 40 % at low doses. Moreover, it is active against the well-known HeLa cancer cell line and various microbes, including Staphylococcus aureus, Enterococcus faecium, and Ustilago maydis.
“It is an intriguing molecule and one that gave us a hard time,” Nicolaou told ChemViews Magazine. “Thanks to the perseverance of my students, it did not escape.” He adds that “The next step in this work will include the design, synthesis, and biological evaluation of analogs of namenamicin and related natural products.”
- Total Synthesis and Full Structural Assignment of Namenamicin,
K. C. Nicolaou, Ruofan Li, Zhaoyong Lu, Emmanuel N. Pitsinos, Lawrence B. Alemany,
J. Am. Chem. Soc. 2018.
https://doi.org/10.1021/jacs.8b04592
Reference
- [1] Namenamicin, a New Enediyne Antitumor Antibiotic from the Marine Ascidian Polysyncraton lithostrotum,
Leonard A. McDonald, Todd L. Capson, Girija Krishnamurthy, Wei-Dong Ding, George A. Ellestad, Valerie S. Bernan, William M. Maiese, Piotr Lassota, Robert A. Kramer, Chris M. Ireland,
J. Am. Chem. Soc. 1996, 118, 10898–10899.
https://doi.org/10.1021/ja961122n


![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)
