Tropane alkaloids have received a great attention because of their potent biological activities, and some of them, such as atropine, cocaine, and scopolamine, have been used as efficient pharmaceuticals. In addition, polycyclic tropanes have also received a lot of attention. However, identifying and supplying suitable complex polycyclic tropane frameworks is challenging.
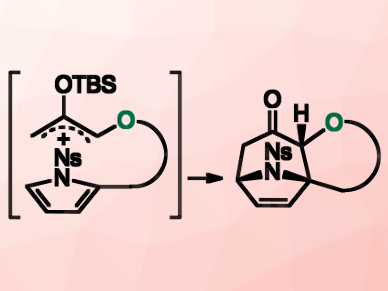
An intramolecular [4+3] cycloaddition reaction of pyrroles with oxyallyl cations is one of the most powerful synthetic approaches to polycyclic tropane frameworks. However, such cycloaddition reactions are difficult due to the instability of the oxyallyl precursor.
Kosuke Namba, Tokushima University, Japan, and colleagues have developed a single step method for the construction of complex polycyclic tropanes with high yields and high stereoselectivities. The team used an in situ formation of the precursor. The condensation of an alcohol with 2-(silyloxy)-acrolein derivatives generates an unstable oxyallyl cation. This reaction is followed by the intramolecular [4+3] cycloaddition reaction of N‐nosyl‐pyrrole with the oxyallyl cation (pictured).
The computational studies suggested that the intramolecular cycloadditon reaction proceeds through an unexpected step-wise mechanism. The cascade reaction readily provides various complex frameworks in three dimensions. According to the researchers, their work contributes to the development of novel pharmaceuticals.
- Direct Synthesis of Polycyclic Tropinones by a Condensation-[4+3]-Cycloaddition Cascade Reaction,
Tsubasa Okamoto, Miki Shibata, Sangita Karanjit, Atsushi Nakayama, Masahiro Yoshida, Kosuke Namba,
Chem. Eur. J. 2018.
https://doi.org/10.1002/chem.201802011