An estimated 20 % of all pharmaceuticals contain fluorine. The element is popular in the pharmaceutical industry because fluorine substituents can alter the physicochemical properties of a drug. For example, they can improve the stability, change binding affinities, and increase the membrane permeability. However, fluorination processes are often elaborate and costly. Ryan Gilmour and colleagues, University of Münster, Germany, have found that a sustainable and elegant fluorination reaction is possible with the help of iodine catalysis.
Selective Fluorination
In nature, the fluorine atom is a rare guest in biomolecules. This might be the reason why enzymes have a hard time degrading fluorine-containing compounds. This is also why pharmacists like this element: Being as small as hydrogen, one fluorine atom in a compound may render the drug more resistant against metabolic degradation, more lipophilic, or add other qualities.
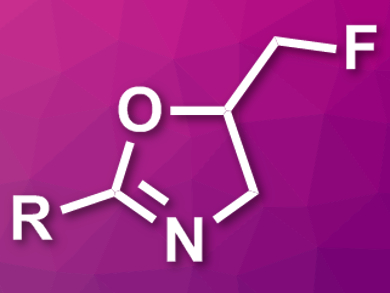
“Fluorine allows you to prepare materials with very unusual properties,” says Ryan Gilmour. His group had previously developed a difluorination strategy for terminal olefins with the help of a hypervalent iodine catalyst [1]. With an extended version of the strategy, the researchers have now prepared fluoromethylated oxazolines (pictured) by fluorocyclization. Fluoromethylated oxazolines are pharmacologically valuable oxazoline bioisosteres. Bioisosteres are chemical compounds with similar biological properties.
Compounds containing an oxazoline moiety often have antibiotic, antitumor, or antifungal activities. Oxazoline is a five-membered heterocycle that can be prepared through an intramolecular ring-closure reaction of carboxamides. This reaction works if a carbocation in β-position to the nitrogen can be generated, which is then attacked by the oxygen of the carboxamide. Since a cation is also involved in the difluorination strategy, the scientists anticipated that fluoromethylated oxazolines would be accessible by a fluorocyclization reaction. This approach could outperform other routes by its speed and efficiency and is catalyzed by iodine.
Hypervalent Iodine Catalysis
Iodine is an environmentally friendly, relatively inexpensive element, which has not yet been fully acknowledged by the industry as a versatile and sustainable catalyst. The redox chemistry of hypervalent iodine is reminiscent of that of transition metals. For example, the iodine(I)–iodine(III) transition shares many aspects of the palladium(0)–palladium(II) cycle. In the fluorocyclization reaction, oxidation of p-iodotoluene (the iodine(I) species) generates p-iodotoluene difluoride or TolIF2 (the iodine(III) species). HF, introduced as an amine–HF mixture, is also a crucial agent in this transformation. It activates the in situ-generated catalyst by its Brønsted-acidic nature.
According to the team, the iodine–HF-mediated fluorination reaction is a convenient, fast, and cheap way to generate 2-oxazoline bioisosteres. The fact that the reagents are abundant, quite environmentally benign, and relatively inexpensive adds to the reaction’s sustainability. The “green” impression that is often associated with organocatalysis suffers slightly from the use of dichlorinated solvents, Gilmour admits. However, the scope of benzamides that can be transformed is broad, and the fluorine atom introduces interesting stereoelectronic effects leading to distinct product conformations. These aspects, as well as a further expansion of the reaction, need to be explored in more depth.
- Fluorocyclisation via I(I)/I(III) catalysis: a concise route to fluorinated oxazolines,
Felix Scheidt, Christian Thiehoff, Gülay Yilmaz, Stephanie Meyer, Constantin G Daniliuc, Gerald Kehr, Ryan Gilmour,
Beilstein J. Org. Chem. 2018, 14, 1021–1027.
https://doi.org/10.3762/bjoc.14.88
Reference
- Catalytic Difluorination of Olefins,
István Gábor Molnár, Ryan Gilmour,
J. Am. Chem. Soc. 2016, 138, 5004–5007.
https://doi.org/10.1021/jacs.6b01183