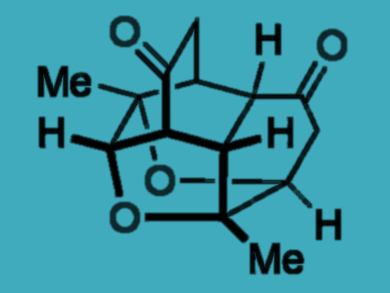
Structurally complex molecules can be expensive and time-consuming to prepare. They require starting materials that are both readily accessible and sufficiently reactive to undergo multiple bond-forming events.
Andrew Lawrence, Fernanda Duarte, and colleagues, University of Edinburgh, UK, have developed a domino reaction sequence by which readily available para-quinols are transformed into highly complex polycyclic molecules. Para-quinols are stable under ambient conditions. However, when treated with an amine catalyst and heated in chloroform, they dimerize efficiently and undergo a series of bond-forming reactions to form tetracycles. A final oxa-Michael addition occurs in the presence of a strong base. It forms a hexacycle containing four new bonds, four new rings, and eight new contiguous stereogenic centers (see scheme).

The tetracycles are accessible in gram-scale quantities, which allows further functionalization of the complex structures. The team suggests that the method could be used to prepare libraries of natural-product-like compounds.
- Bio-inspired Domino oxa-Michael/Diels-Alder/oxa-Michael Dimerization of para-Quinols,
Nicholas J. Green, Catherine A. Connolly, Koen P. W. Rietdijk, Gary S. Nichol, Fernanda Duarte, Andrew L. Lawrence,
Angew. Chem. Int. Ed. 2018, 57, 6198–6202.
https://doi.org/10.1002/anie.201802125