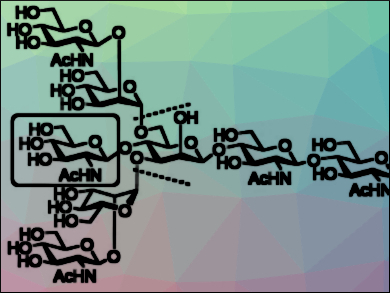
Glycosylation, i.e., attaching carbohydrates, is a common protein modification. Asparagine-linked polysaccharides (N-glycans) are one such type of carbohydrate. They have high structural and bio-functional diversities. Branched N-glycans which contain a bisecting N-acetylglucosamine (GlcNAc, pictured in the box), for example, have various biological functions. A sufficient supply of this type of N-glycan is required to investigate the biological functions of the bisecting GlcNAc.
Koichi Fukase, Osaka University, Japan, and colleagues have synthesized an N-glycan which contains a bisecting GlcNAc (pictured) by a convergent synthetic route via [4+2] and [6+2] glycosylations. This synthetic strategy reduced the number of reaction steps. The key glycosylations were challenging in terms of yields and selectivity due to steric hindrance at the glycosylation site and a lack of neighboring-group participation. The team enhanced the yields of these reactions by stabilizing the oxocarbenium ion intermediate through ether coordination. Glycosyl-donor protecting groups were used to achieve the desired selectivity.
The developed approach provides a universal synthetic strategy for the efficient synthesis of various N-glycans which could accelerate functional studies of these compounds.
- Convergent synthesis of a bisecting GlcNAc-containing N-glycan,
Yoshiyuki Manabe, Hiroki Shomura, Naoya Minamoto, Masahiro Nagasaki, Yohei Takakura, Katsunori Tanaka, Alba Silipo, Antonio Molinaro, Koichi Fukase,
Chem. Asian J. 2018.
https://doi.org/10.1002/asia.201800367