Chemical transformations are typically accomplished by using highly reactive species generated in situ by heat or a catalyst to facilitate the reaction. One intriguing alternative for producing reactive intermediates is the use of light to promote readily accessible starting materials into their excited state, thereby enabling bond activation and the creation of chemical species that are not accessible by conventional methods.
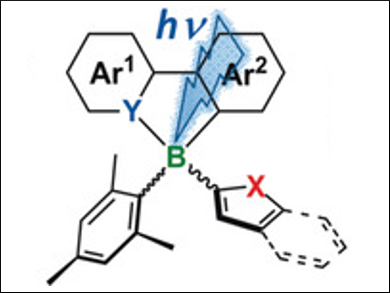
Suning Wang, Queen’s University, Kingston, Ontario, Canada, and Beijing Institute of Technology, China, Quan‐Song Li, Beijing Institute of Technology, and colleagues have found that chiral N,C-chelated boron compounds can selectively and quantitatively activate the C–S/C–O bonds of thienyl and furyl units through a boron insertion reaction upon photoexcitation. This reactivity represents the first example of heteroaromatic C–S bond activation by a main-group species and light.
The products obtained are rare chiral N,B,S- or N,B,O-heterocycles that show intense and tunable luminescence and, thus, have potential applications in optoelectronics. This work provides a new strategy for chemical bond activation and the photochemical production of new species containing main-group elements.
- Photochemical Generation of Chiral N,B,X-Heterocycles by Heteroaromatic C–X Bond Scission (X=S, O) and Boron Insertion,
Soren K. Mellerup, Cally Li, Julian Radtke, Xiang Wang, Quan-Song Li, Suning Wang,
Angew. Chem. Int. Ed. 2018.
https://doi.org/10.1002/anie.201803760