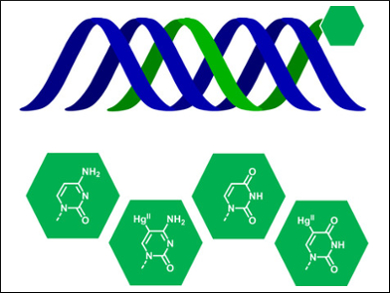
The formation of triple-stranded DNA is supposed to play a role in the regulation of gene expression. Triplex-forming oligonucleotides hold promise as therapeutic agents. They bind to the major groove of double helical DNA, where they inhibit gene expression by forming a local triple helix. However, triplex-forming oligonucleotides typically suffer from relatively low hybridization affinity and, therefore, low stability.
Tuomas Lönnberg and Dattatraya Uttam Ukale, University of Turku, Finland, have demonstrated the stabilization of triple helices by covalently mercurated base moieties. The team synthesized homothymine oligonucleotides with a single 5‐mercuricytosine or 5‐mercuriuracil residue at their termini. UV and circular dichroism (CD) melting experiments were used to monitor the formation and thermal denaturation of the triple helices.
Within an extensive array of double-helical targets, nearly all triplexes were destabilized upon mercuration of the 3′‐terminal residue. However, triplex stabilization by 5‐mercuricytosine was observed with targets bearing a T–A or A–T base pair opposite to the mercurated nucleobase. Although the origin of the stabilization remains unclear, metalated nucleobases could become a valuable tool to increase the stability of triplex-forming oligonucleotides.
- Triplex Formation by Oligonucleotides Containing Organomercurated Base Moieties,
Dattatraya Uttam Ukale, Tuomas Lönnberg,
ChemBioChem 2018.
https://doi.org/10.1002/cbic.201800112