Heparin is a highly sulfated glycosaminoglycan of natural origin and is very commonly used as an anticoagulant and antithrombotic drug. Despite its clinical use for a century, the biological properties of heparin are not completely understood. Chemoenzymatic synthesis has provided access to highly tuneable oligosaccharides, which opens up opportunities for characterizing heparin–protein interactions.
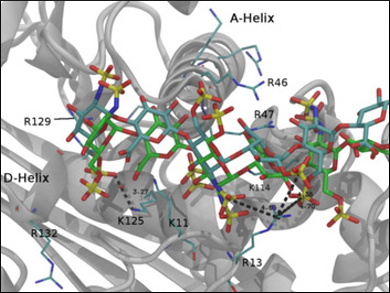
Marco Guerrini, Ronzoni Institute, Milan, Italy, and colleagues have studied an example of a new antithrombin-binding heparin oligosaccharide prepared by chemoenzymatic synthesis. The compound can activate the coagulation inhibitor antithrombin despite a lack of 2-O-sulfation at the iduronic acid residue. This is a remarkable finding given that the most common disaccharide in heparin is composed of a 2-O-sulfated iduronic acid and a 6-O-sulfated, N-sulfated glucosamine.
The availability of new oligosaccharide models, coupled with up to date NMR spectroscopy techniques and molecular dynamics (MD) simulations, could contribute a full understanding of the complex physiological role of heparin.
- Recognition and Conformational Properties of an Alternative Antithrombin Binding Sequence Obtained by Chemoenzymatic Synthesis,
Eduardo Stancanelli, Stefano Elli, Po-Hung Hsieh, Jian Liu, Marco Guerrini,
ChemBioChem 2018.
https://doi.org/10.1002/cbic.201800095