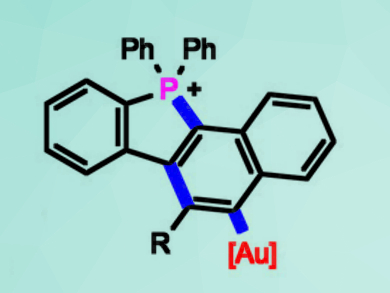
In homogeneous gold(I) catalysis, a large part of the research is concerned with diyne cyclizations. One cyclization approach is the addition of nucleophiles, e.g., amines. The mechanism was proposed to follow a hydroamination-hydroarylation cascade. However, important intermediates had not been isolated so far.
A. Stephen K. Hashmi, University of Heidelberg, Germany, and King Abdulaziz University (KAU), Jeddah, Saudi Arabia, Yana Vaynzof, University of Heidelberg, and colleagues have developed a diyne cyclization cascade comprising the known nucleophilic addition of phosphanes. Due to the electron-deficient nature of the generated phosphonium moiety, it was possible to isolate key-intermediates (example pictured).
The results of the study indicate a cascade through phosphinoauration-6-endo–dig-cyclization. Hydroarylation, however, was not observed. The gold(I) center was found to be exchanged during the process, which led to the proposal of a new type of mechanism. This “gold-exchange mechanism” could also contribute to related cascades with electron-poor substrates.
The phosphoniumfluorenes produced by the reaction show low reduction potentials, which could make them useful in materials science. The compounds were tested as a hole-blocking layer in perovskite solar cells. The efficiency of the device increased from 10.7 % without the material, and to 14.2 % with the phosphoniumfluorene.
- The Gold(I)-Mediated Domino Reaction to Fused Diphenyl Phosphoniumfluorenes: Mechanistic Consequences for Gold-Catalyzed Hydroarylations and Application in Solar Cells,
Sebastian Arndt, Jan Borstelmann, Rebeka Eshagh Saatlo, Patrick W. Antoni, Frank Rominger, Matthias Rudolph, Qingzhi An, Yana Vaynzof, A. Stephen K. Hashmi,
Chem. Eur. J. 2018.
https://doi.org/10.1002/chem.201800460