Chemical reactions involve forming and breaking bonds. One type of bond-breaking process, called bond homolysis (BHo), can provide crucial information for understanding the nature of chemical bonding. Until now, only two potential energy minima were thought to exist in carbon–carbon BHo: a σ-bonding isomer and a singlet-diradical isomer.
Manabu Abe, Hiroshima University, Japan, Hui-Hsu Gavin Tsai, National Central University, Taoyuan City, Taiwan, and colleagues have studied bond-homolysis reactions in1,4-diarylbicyclo[2.1.0]pentane derivatives. The team conducted emission spectroscopic analyses of the compounds at 77 K in a 3‐methylpentane matrix. They used photoexcitation to induce bond-breaking.
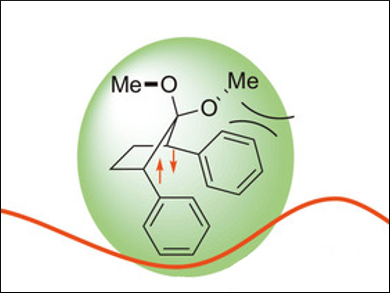
The researchers found a third isomer in the excited states of the compounds: a puckered singlet diradical (pictured) which shows long-wavelength fluorescence beyond 460 nm. The team also used quantum chemical calculations to study the potential energy surface of BHo processes and confirm their experimental observations.
- Unusually Long-Wavelength Emissions of Cyclopropanes: New Insight into C–C Bond Homolysis,
Manabu Abe, Kousei Kanahara, Norito Kadowaki, Chun-Jui Tan, Hui-Hsu Gavin Tsai,
Chem. Eur. J. 2018.
https://doi.org/10.1002/chem.201800671