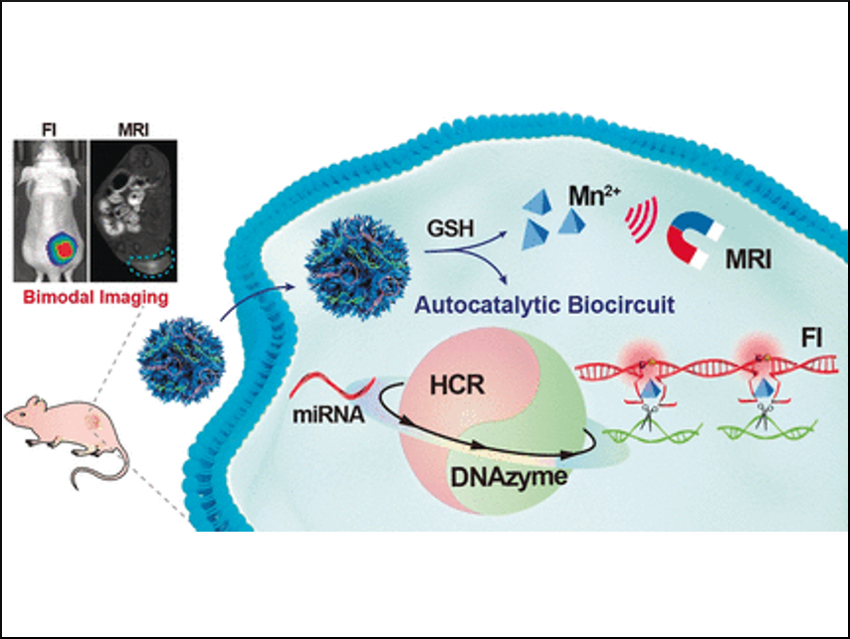
Cancer Diagnostics
A good indicator of dysregulation in live cells is a change in their RNA expression. MicroRNA (miRNA), a special type of RNA, is considered a biomarker for carcinogenic cells. Lin Li, Nanjing Tech University, China, Shao Q. Yao, National University of Singapore, Wei Huang, Nanjing Tech University and Northwestern Polytechnical University, Xi’an, China, and colleagues have found a way to amplify miRNA in live tumor cells for bioimaging. Their assay is based on a robust cellular autocatalytic biocircuit triggered by synthetic DNA and nanoparticles.
Diagnosing cancer before a tumor becomes visible has been one of the long-standing goals in medicine. One of the biomarkers for carcinogenicity in a cell is its RNA expression pattern or, more precisely, the change in RNA expression, which causes metabolic degeneration. There are many types of RNA, among which a short noncoding RNA called miRNA promotes or impedes the translation of nucleus-encoded genetic information into protein. Accordingly, the detection of a changed miRNA expression profile is thought to be a reliable indication of the degeneration of a cell.
However, the detection of a particular miRNA is difficult because it is present in the cell only in tiny amounts and must be amplified and connected to a signaling entity, such as a fluorescence dye, for visualization. The researchers have discovered a suitable amplification–detection mechanism for miRNA, which relies on an autocatalytic biocircuit activated by synthetic DNA, leading to a strong fluorescence signal that flags tumor cells.
Amplification and Imaging of microRNA
RNA is usually synthesized in the nucleus of the cell and transported to the cytoplasm where it conveys genetic information. However, when synthetic DNA is present in the cytoplasm, RNA can bind to a matching nucleotide sequence of the DNA strand; a fact that is exploited in, for example, antiretroviral treatment to silence viral RNA expression. The team did the opposite: By matching synthetic DNA strands with miRNA, they triggered an autocatalytic amplification circuit—called autocatalytic DNAzyme biocircuit—to form DNA–miRNA assemblies. These assemblies grew further to form DNAzyme nanowires that carry the fluorescence dyes.
After administering the DNAzyme detection kit, the team observed bright fluorescence in a mouse model at the location where a tumor was developing.
To make the DNAzyme enter the tumor cells, the researchers used nanoparticles—tiny parcels that can deliver drugs and other molecular freight to the cells—made of manganese dioxide with a honeycomb-like structure. According to the team, this composition and architecture has the advantage that the nanoparticle can be readily activated by glutathione, which is a chemical found in abundance in tumor cells. Another advantage is that the released manganese ions would sustain the autocatalytic DNAzyme biocircuit.
The researchers emphasize that their self-enhanced bioimaging system could be developed as a powerful tool to visualize tumor cells with biomarkers. This is especially promising as many different miRNAs can be selectively targeted to investigate different cancers or other cell dysfunction.
- Rational Design of a Two‐Photon Fluorogenic Probe for Visualizing Monoamine Oxidase A Activity in Human Glioma Tissues,
Haixiao Fang, Hang Zhang, Lin Li, Yun Ni, Riri Shi, Zheng Li, Xuekang Yang, Bo Ma, Chengwu Zhang, Qiong Wu, Changmin Yu, Naidi Yang, Shao Q. Yao, Wei Huang,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202000059