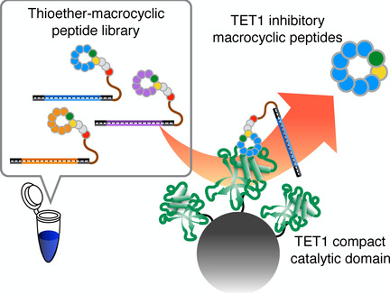
Members of the ten-eleven translocation (TET) protein family have an important role in 5-methylcytosine demethylation in genomic DNA. DNA methylation/demethylation contributes to the regulation of gene expression at the transcriptional level. The ten-eleven translocation (TET) protein family consists of three isoforms (TET1/2/3).
Hiroaki Suga, University of Tokyo, Japan, and colleagues have reported TET1 inhibitors based on a thioether macrocyclic peptide scaffold. The team has discovered the inhibitors by using the random nonstandard peptide integrated discovery (RaPID) system, an emerging technique used for affinity screening of drug-like compounds by synthesis of extremely diverse libraries of non-canonical macrocyclic peptides (read more on RaPID in a recent review by H. Suga [1]). These peptides inhibit the catalytic domain of TET1 (TET1CD) with an IC50 as low as 1.1 µM. One of the synthesized peptides inhibits TET1CD over another TET isoform (TET2CD) with 10-fold selectivity, a remarkable finding given the close structures of the two isoforms of TET.
According to the researchers, these finding might help to further improve the potency and the selectivity of TET1 inhibitors, which would reduce potential side-effects caused by unwanted inhibition of other TET isoforms.
- Thioether Macrocyclic Peptides Selected against TET1 Compact Catalytic Domain Inhibit TET1 Catalytic Activity,
Kosuke Nishio, Roman Belle, Takayuki Katoh, Akane Kawamura, Toru Sengoku, Kazuharu Hanada, Noboru Ohsawa, Mikako Shirouzu, Shigeyuki Yokoyama, Hiroaki Suga,
ChemBioChem 2018.
https://doi.org/10.1002/cbic.201800047
[1] Toby Passioura, Hiroaki Suga, A RaPID way to discover nonstandard macrocyclic peptide modulators of drug targets, Chem. Commun. 2017, 53, 1931. https://doi.org/10.1039/C6CC06951G