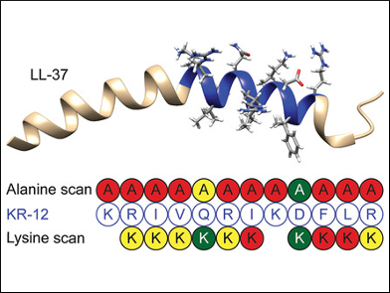
The widespread overuse of antibiotics has contributed to the emergence of many resistant antibacterial strains. To efficiently treat infections caused by those bacteria, new antibiotics are urgently needed. This has put a spotlight on antimicrobial peptides. The human host defense peptide LL-37 is a broad-spectrum antibiotic. From this peptide, a minimal peptide (KR-12, residues 18–29 in LL-37) has been identified which shows antibacterial activity with decreased cytotoxicity.
Ulf Göransson, Uppsala University, Sweden, and colleagues have studied the structure and function of KR-12 by substituting individual amino acids in its sequence with either alanine or lysine. Several synthesized KR-12 analogs show improved characteristics against different human pathogens (Staphylococcus aureus, Pseudomonas aeruginosa, and Candida albicans).
The results show relationships between charge, hydrophobicity, and the position of amino acid residues and the peptides’ potency and selectivity. Mechanistic assays suggest membrane permeabilization as the main mode of action. This could aid the further exploration and design of antimicrobial peptides.
- Alanine and Lysine Scans of the LL-37-Derived Peptide Fragment KR-12 Reveal Key Residues for Antimicrobial Activity,
Sunithi Gunasekera, Taj Muhammad, Adam A. Strömstedt, K. Johan Rosengren, Ulf Göransson,
ChemBioChem 2018.
https://doi.org/10.1002/cbic.201700599