Protein–protein interactions (PPs) are central to most biological processes and are, therefore, attractive therapeutic targets. The targeting of those interactions, however, remains a challenge. Protein-Observed Fluorine NMR (PrOF NMR) spectroscopy is an emerging technique for screening and characterizing small molecule-protein interactions. The choice of which amino acid to label for PrOF NMR can be critical for analysis.
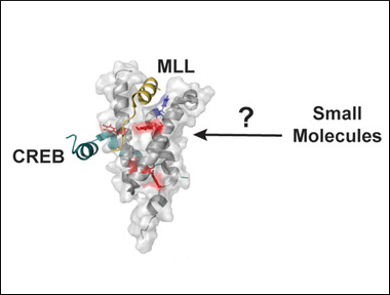
William C. K. Pomerantz, University of Minnesota, Minneapolis, USA, and colleagues have reported the first use of a protein containing two different fluoroaromatic amino acids for NMR studies. The team used the KIX domain of the CREB Binding Protein (CBP/p300) as a model system. They examined KIX binding interactions with previously reported and novel small molecules and identified a new binding site distinct from those used by native transcription factors. Such a new binding site provides an opportunity to regulate KIX PPIs allosterically, either positively or negatively.
According to the authors, metabolic labeling with multiple fluorinated amino acids provides useful probes for further studying ligand binding.
- Dual Labeling of the CBP/p300 KIX Domain for 19F NMR Leads to Identification of a New Small-Molecule Binding Site,
Clifford T. Gee, Keith E. Arntson, Edward J. Koleski, Rachel Lynn Staebell, William C. K. Pomerantz,
ChemBioChem 2018.
https://doi.org/10.1002/cbic.201700686