The ancient Egyptians already used aluminum crystals as antiperspirants. The ancient Romans used a mixture of charcoal and goat fat as deodorant. In the 19th century, lime solutions or potassium permanganate were used. These substances work disinfecting. The first commercial deodorant was patented by Edna Murphey in Philadelphia, PA, USA, in 1888. Today, they are an everyday part of life. So let us look at how they work from a chemical perspective.
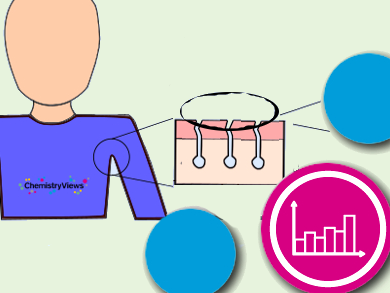
Where Does Body Odor Come From?

What is the Difference Between Deodorants and Antiperspirants? How Do Deodorants Work?

There are also “mixed” products with both antibacterial and antiperspirant properties.
How Do Antiperspirants Work?
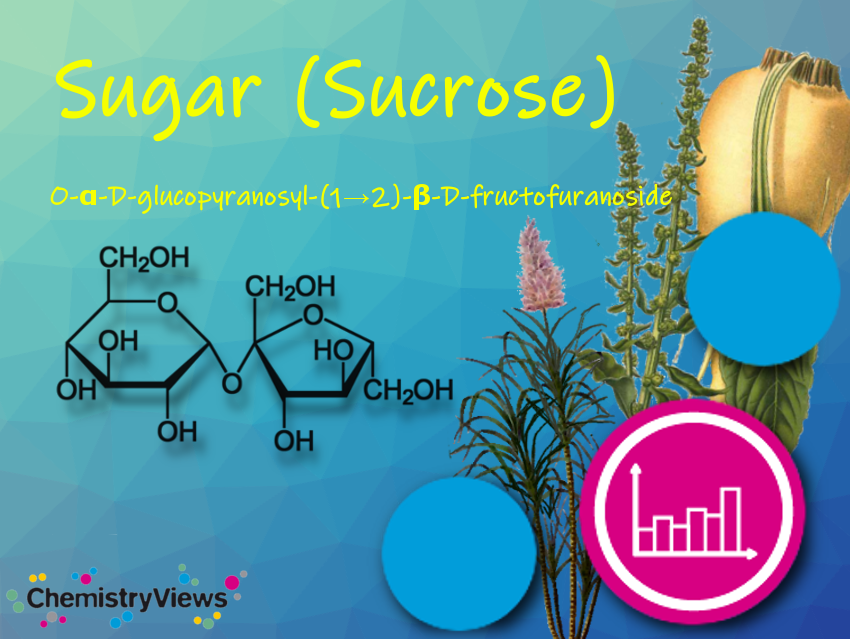
Aluminum or zirconium compounds, such as aluminum chlorohydrate, react with water in the glands to form gel-like plugs of aluminium hydroxide that block the sweat glands:
.jpg)
Which Ingredients Do Deodorants and Antiperspirants Contain?
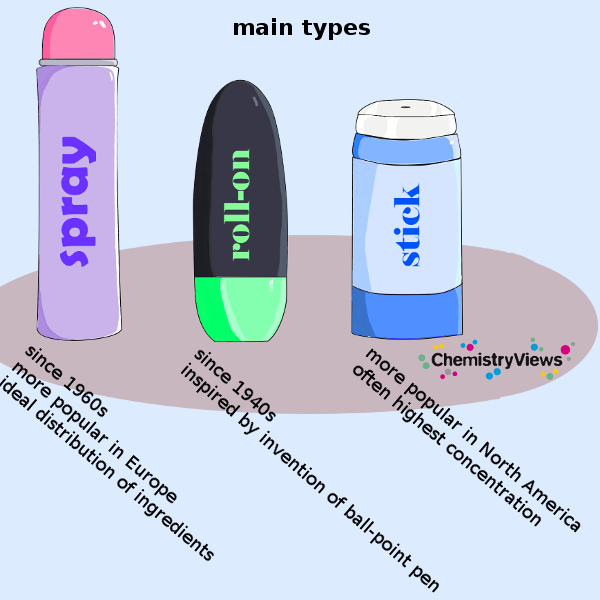
Types of Deodorants and Antiperspirants
Some Health and Environmental Risks
.jpg)
Sources
- John Emsley, Chemistry at Home: Exploring the Ingredients in Everyday Products, Royal Society of Chemistry, London, UK, 2015. ISBN: 978-1-84973-940-5
- Juanshu Shen, Véronique Nardello-Rataj, Deodorants and antiperspirants: Chemistry under arms, L’Act. Chim. 2009, 331, 8. https://doi.org/
- S. D. Williams, W. H. Schmitt, Chemistry and Technology of the Cosmetics and Toiletries Industry, Springer, New York, 1992. ISBN-13: 978-9401050074
- European Commission DG Health and Food Safety, Scientific Opinion on Aluminium in cosmetic products published today, ec.europa.eu, 2014.
- Scientific Committee on Consumer Safety (SCCS), Scientific Opinion on Aluminium in cosmetic products published today, European Commission 2014.
.png)