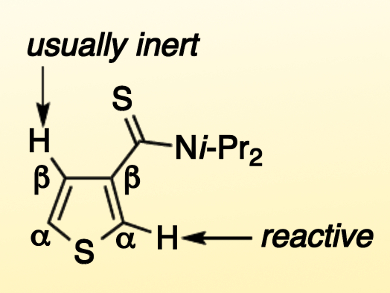
Thiocarbonyl groups are potential strong directing groups for transition-metal catalysis. However, the decomposition of the thiocarbonyl groups and the reaction of the metals with the released sulfur species usually leads to deactivation of the catalyst, and thus, there are few productive examples of this type of reaction.
Fumitoshi Shibahara, Toshiaki Murai, and Yusuke Asai, Gifu University, Japan, have developed a Pd-catalyzed chelation-assisted β-selective direct C–H bond arylation of a thienylthioamide. The team had previously found that a Pd–phenanthroline system avoids decomposition processes, and a catalytic direct C–H bond arylation of 3-thienylthioamide selectively proceeds at the 2-position (α-position) of thiophene through a chelation-assisted manner with high efficiency [1].
The researchers studied if this chelation effect could potentially overcome the fundamental reactivity of thiophene, i.e., a much higher reactivity at the α-positions than the β-positions. It turned out that the reaction of 2-thienylthioamide, which has only a C–H bond next to a thiocarbonyl group at the β-position, proceeds selectively at that position. This finding indicates a promising future for the usage of thiocarbonyl groups in catalysis.
- Chelation-assisted β-selective direct C–H bond arylation of 2-thienylthioamide catalyzed by Pd-1,10-phenanthroline complexes,
Fumitoshi Shibahara, Yusuke Asai, Toshiaki Murai,
Asian J. Org. Chem. 2018.
https://doi.org/10.1002/ajoc.201800160This article is part of a Special Issue on C–H activation
Asian J. Org. Chem. 2018, 7 (7).
Reference
- [1] Direct C–H Bond Arylation of Thienyl Thioamides Catalyzed by Pd–Phenanthroline Complexes,
Takayuki Yamauchi, Fumitoshi Shibahara, Toshiaki Murai,
Org. Lett. 2015, 17, 5392–5395.
https://doi.org/10.1021/acs.orglett.5b02742