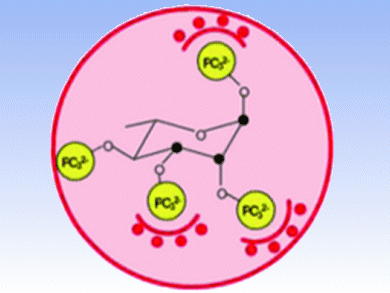
There are a variety of molecules — both endogenous and synthetic — that help hemoglobin (Hb) deliver its oxygen cargo. Such allosteric effectors work by binding the Hb tetramer in a manner that decreases its affinity for O2. For diseases involving hypoxic conditions, including cancer and cardiovascular disease, compounds that restore normoxia by promoting O2 release from Hb have great therapeutic potential.
An international research team led by Jean-Marie Lehn, University of Strasbourg, France, reports the synthesis of a series of perphosphorylated pentopyranose and pentofuranose derivatives as allosteric effectors of Hb. Some of these compounds show Hb binding affinity and efficacy in O2 release on par with the most potent allosteric effector known. The collaboration’s detailed analysis and characterization sheds light on the key structural aspects that impart these molecules with the ability to facilitate O2 delivery.
- Polyphosphates and Pyrophosphates of Pentopyranoses and Pentofuranoses as Allosteric Effectors of Human Hemoglobin: Synthesis, Molecular Recognition, and Oxygen Release
K. C. Fylaktakidou, C. D. Duarte, R. Jogireddy, A. E. Koumbis, C. Nicolau, J.-M. Lehn,
ChemMedChem 2011, 6(8).
DOI: 10.1002/cmdc.201100110