Sapphyrins (Pentaphyrin(1.1.1.1.0)s) are the first expanded porphyrins to have been discovered by Woodward et al. [1]. β-Substituted sapphyrins are often used as versatile anion-binding agents and their structures have been analyzed in their protonated forms. Consequently, meso-substituted sapphyrins are sometimes insufficiently stable. For these reasons, the crystal structures of non-inverted neutral sapphyrins have not been obtained yet. In addition, their coordination chemistry has not been studied in sufficient detail.
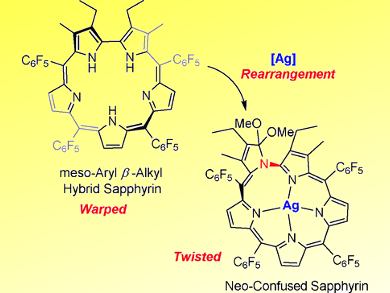
Tomoki Yoneda and Saburo Neya, Chiba University, Japan, and colleagues have synthesized a new meso-aryl and β-alkyl substituted sapphyrin. Its non-inverted and warped structure in its neutral state was analyzed by X-ray crystallography. The structure of the sapphyrin moiety was flattened by double RhI(CO)2 metallation and aromaticity of the complex was enhanced by the Rhodium(I) metalation. Moreover, metalation with silver acetate in CH2Cl2/methanol caused a rearrangement into a neo-confused (C–N-bonded) sapphyrin–AgI complex with a twisted topology.
- Stable meso -Aryl β-Alkyl Hybrid Sapphyrin with a Warped π-Conjugation Circuit and Neo-Confused Sapphyrin-Silver(I) Complex,
Daiki Mori, Tomoki Yoneda, Tyuji Hoshino, Saburo Neya,
Chem. Asian J. 2018.
https://doi.org/10.1002/asia.201800286
- [1] Sapphyrins: novel aromatic pentapyrrolic macrocycles,
Victor J. Bauer, Derrick L. J. Clive, David Dolphin, John B. Paine III, Francis L. Harris, Michael M. King, John Loder, Shen Wei Chien Wang, Robert Burns Woodward,
J. Am. Chem. Soc. 1983, 105 (21), 6429–6436.
https://doi.org/10.1021/ja00359a012
.JPG)



