Monocarba-closo-dodecaborate (C1-carborane anion) has interesting molecular properties, including excellent anion stability, 3D aromaticity (σ aromaticity), and structural uniqueness. Thus, this scaffold is potentially attractive as a core of functional molecules. However, the use of this molecule is still rare due to a lack of methods for its functionalization.
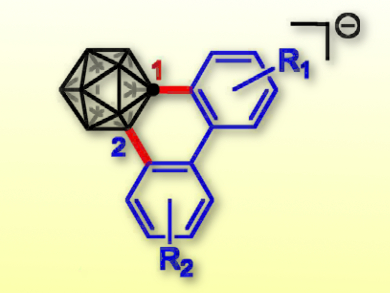
Ryo Takita, RIKEN, Saitama, Japan, Masanobu Uchiyama, RIKEN and University of Tokyo, Japan, and colleagues have developed an efficient one-pot annulation reaction for biaryl-fused derivatives of this molecule. A variety of anionic “3D-triphenylene” derivatives were prepared by a sequential carbon–carbon/carbon–boron bond formation.
The process proceeds via a copper-mediated C–C cross-coupling reaction, followed by a palladium-catalyzed intramolecular arylation via inert aromatic B–H bond disconnection without pre-functionalization of the C1-carborane cage. The fused derivatives are expected to present unique conjugation behaviors.
- One-pot Annulation for Biaryl-fused Monocarba-closo-dodecaborate through Aromatic B−H Bond Disconnection,
Gaku Akimoto, Mai Otsuka, Kazunori Miyamoto, Atsuya Muranaka, Daisuke Hashizume, Ryo Takita, Masanobu Uchiyama,
Chem. Asian J. 2018.
https://doi.org/10.1002/asia.201800053