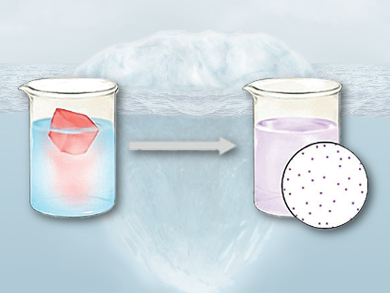
The kinetic and thermodynamic aspects of wet chemistry are influenced by physical processes that occur when different solutions are mixed. Nucleation of reaction products is suppressed by low concentrations of reactants. However, it is challenging to prepare atomically dispersed products using solution-phase synthesis.
Hui Wu, Tsinghua University, Li-Min Liu, Beihang University, both Beijing, China, and colleagues have developed a method by which a frozen solution of a metal salt (AgNO3), mounted on a piece of ice, is thawed within a reducing solution (NaBH4) at 0 °C. The method allows the slow release and reduction of metal ions in a solution-based reaction. This suppresses product nucleation and aggregation phenomena, resulting in atomically dispersed metals. .
Using the method, the team prepared carbon films on which different types of metals were atomically dispersed, including cobalt, nickel, copper, ruthenium, rhodium, palladium, silver, osmium, iridium, platinum, and gold. The technique can be extended to other solution-based syntheses and provides a new and general method for modulating the kinetics, thermodynamics, and reactant diffusion of processes such as reduction, precipitation, hydrolysis, and displacement reactions.
- Ice Melting to Release Reactants in Solution Syntheses,
Hehe Wei, Kai Huang, Le Zhang, Binghui Ge, Dong Wang, Jialiang Lang, Jingyuan Ma, Da Wang, Shuai Zhang, Qunyang Li, Ruoyu Zhang, Naveed Hussain, Ming Lei, Li-Min Liu, Hui Wu,
Angew. Chem. Int. Ed. 2018.
https://doi.org/10.1002/anie.201711128