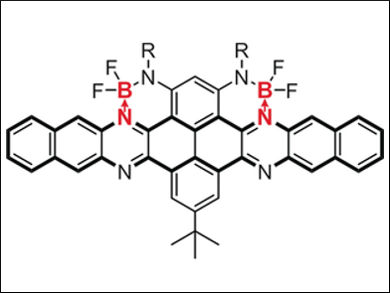
Organic semiconductors are very important as materials for various optoelectronic devices. However, the development of n-type (electron-transporting) organic semiconductors lags far behind that of p-type (hole-transporting) organic semiconductors.
Chuandong Dou, Jun Liu, Changchun Institute of Applied Chemistry, Chinese Academy of Sicences, and colleagues have developed a way to use boron-nitrogen coordination bonds (B←N) to design n-type organic semiconductors. The researchers synthesized a new kind of n-type acene, namely two azaacenes containing B←N units (pictured). These B←N-containing azaacenes have low-lying LUMO (lowest unoccupied molecular orbital) energy levels and n-type character.
Solution-processed organic field-effect transistors (OFETs) based on one such azaacene displayed unipolar n-type characteristics with an electron mobility of 0.21 cm2 V–1 s–1. In addition, the B←N units significantly perturbed the electronic structures of azaacenes, resulting in unique LUMOs delocalized on the acene skeletons and a decreased aromaticity of the B←N-adjacent rings. According to the scientists, this research not only provides a new strategy to develop n-type organic semiconductors, but also deepens our understanding of the effects of B←N units on π-conjugated systems.
- n-Type Azaacenes Containing B←N Units,
Yang Min, Chuandong Dou, Hongkun Tian, Yanhou Geng, Jun Liu, Lixiang Wang,
Angew. Chem. Int. Ed. 2018, 57, 2000–2004.
https://doi.org/10.1002/anie.201712986