The addition of aryllithium reagents to sterically congested polycyclic quinones provides access to twistacenes, compounds with interesting and potentially useful electrical and chiroptical properties. This synthetic methodology is an alternative to the traditional “benzyne” approach and provides advantages such as shorter syntheses and improved overall yields.
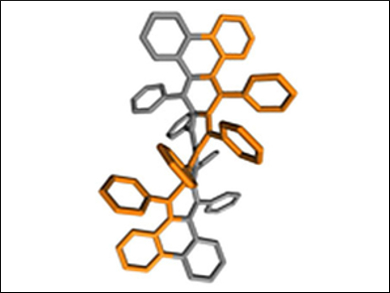
Kathleen V. Kilway, University of Missouri, Kansas City, USA, and colleagues have prepared 9,10,11,12,21,22,23,24-octaphenyltetrabenzo[a,c,n,p]hexacene (pictured), a helical acene with a longitudinal twist of 184°. It is the largest end-to-end twist in an acene to be recorded to date. This twist was verified by X-ray crystallography. The key steps in the synthesis of this compound were the addition of phenyllithium to a sterically congested polycyclic quinone, followed by the SnCl2-mediated reduction of the diol intermediate.
According to the researchers, the successful synthesis of this hexacene suggests that the construction of even longer and more twisted acenes may be easier to achieve than previously expected.
- Synthesis and Structure of a Longitudinally Twisted Hexacene,
Robert G. Clevenger, Bharat Kumar, Elizabeth M. Menuey, Kathleen V. Kilway,
Chem. Eur. J. 2018.
https://doi.org/10.1002/chem.201705676