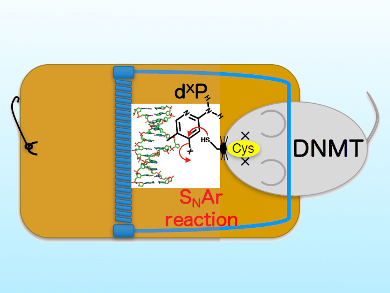
DNA methylation is an important regulator of gene expression. It is catalyzed by ubiquitous enzymes called DNA methyltransferases. Tumor cells show altered DNA methylation patterns. Therefore, inhibition of DNA methyltransferases could be an an effective strategy to treat various cancers. One important part of the methylation machinery is the so called DNA cytosine-5 methyltransferase (DNMT), which is responsible for generating the modified DNA base 5-methylcytosine.
Kousuke Sato, Health Sciences University of Hokkaido, Japan, and colleagues have designed, synthesized, and studied artificial 2-amino-4-halopyridine-C-nucleosides (dXP) and oligodeoxyribonucleotides containing dXP as as inhibitors of DNA cytosine-5 methyltransferases. The new dXP nucleoside forms a covalent bond in the active site of DNA cytosine-5 through a nucleophilic aromatic substitution (SNAr) reaction (pictured) and therefore efficiently inhibits the enzyme.
The results suggest that dXP and oligodeoxyribonucleotides containing dXP could serve as potential nucleic acid drugs in cancer chemotherapy and as useful chemical probes for epigenetic studies.
- Mechanism-based Inhibitor of DNA Cytosine-5 Methyltransferase (DNMT) via a SNAr Reaction with an Oligodeoxyribonucleotide Containing 2-Amino-4-Halopyridine-C-Nucleoside (dXP),
Kousuke Sato, Yuma Kunitomo, Yukiko Kasai, Shohei Utsumi, Isao Suetake, Shoji Tajima, Satoshi Ichikawa, Akira Matsuda,
ChemBioChem 2018.
https://doi.org/10.1002/cbic.201700688