Flagellin is the main structural component of the bacterial flagellum, the appendage responsible for the mobility, adhesion, and invasiveness of bacteria. It has been shown that immunization with flagellin can protect against bacterial infections. Therefore, flagellin has been used as a carrier in conjugate vaccines with a variety of antigens. However, the necessary site-selective modification of flagellin still remains a challenge.
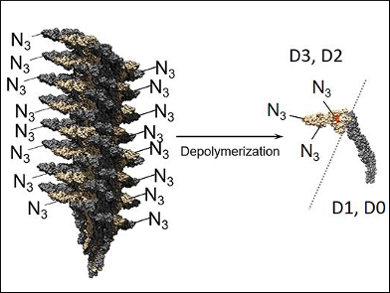
Jim-Min Fang, National Taiwan University, Taipei, and colleagues have developed a strategy for the selective modification of the variable flagellin D2 and D3 domains (pictured) that are exposed on the surface of the flagellar filament. These domains are functionalized with azido groups using a diazo-transfer reaction with imidazole-1-sulfonyl azide. The D0 and D1 domains for the activation of the toll-like receptor 5 (TLR5) are inside the filament under the steric self-protection strategy during the reaction.
This approach is practical for scaled-up preparation at the milligram level across a variety of bacterial strains and species within a short period, without genetic engineering or complicated operations. It allows the production of the immunogenic flagellin as a self-adjuvanting carrier. This site-selective modification method could help with the development of conjugate vaccines against various bacterial pathogens, including multidrug-resistant bacteria.
- Site-Selective Functionalization of Flagellin by Steric Self-Protection: A Strategy to Facilitate Flagellin as a Self-Adjuvanting Carrier in Conjugate Vaccine,
Chi-Jiun Peng, Hsiu-Ling Chen, Cheng-Hsun Chiu, Jim-Min Fang,
ChemBioChem 2018.
https://doi.org/10.1002/cbic.201700634