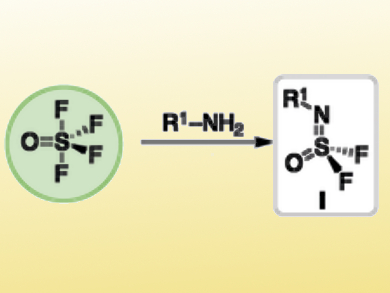
Many sulfur(VI) fluoride compounds contain S–F bonds that are thermodynamically stable and redox-silent. However, S–F exchange occurs easily with specific nucleophiles.
Barry Sharpless, The Scripps Research Institute, La Jolla, CA, USA, and colleagues have developed a click reaction named SuFEx (Sulfur Fluoride Exchange) that sequentially replaces the four S–F bonds of thionyl tetrafluoride (SOF4) to efficiently form diverse sulfur compounds. A primary amine (R1–NH2) is reacted with SOF4 to produce tetrahedral iminosulfur oxydifluorides (R1–N=SOF2, pictured). The remaining two fluoride groups can be exchanged sequentially with a range of nucleophiles, such as amine and aryloxy nucleophiles, to form the desired products.
The researchers created S–C bonds from these functionalized S(VI) species by selectively substituting one of the two remaining F atoms with an aryllithium reagent. The resulting sulfonimidoyl fluorides undergo a further nucleophilic substitution to produce a diverse array of useful functional groups, including sulfoximines, sulfonimidamides, and sulfonimidates.
- SuFEx Chemistry of Thionyl Tetrafluoride (SOF4) with Organolithium Nucleophiles: Synthesis of Sulfonimidoyl Fluorides, Sulfoximines, Sulfonimidamides, and Sulfonimidates,
Bing Gao, Suhua Li, Peng Wu, John E. Moses, K. Barry Sharpless,
Angew. Chem. Int. Ed. 2018.
https://doi.org/10.1002/anie.201712145