The oxidative addition of organic halides to palladium(0) complexes is a fundamental step in the catalytic cycle of several widely used Pd-catalyzed transformations, such as Suzuki couplings, Heck reactions, and Buchwald-Hartwig aminations. In the case of haloarenes, the textbook mechanism involves the insertion of a single Pd(0) center in the carbon-halogen bond.
Luca Alessandro Perego, École Normale Supérieure and CNRS-Chimie ParisTech, Paris, France, Ilaria Ciofini, CNRS-Chimie ParisTech, Laurence Grimaud, École Normale Supérieure, and colleagues have found that two palladium centers can collaborate in the activation of C–I bonds. Conductimetric monitoring of the reaction of iodoarenes with Pd(0) complexes featuring nitrogen ligands showed a second-order dependence of the reaction rate on the concentration of Pd(0).
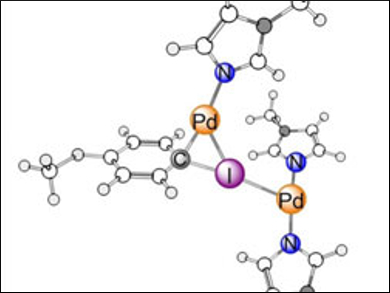
Density functional theory (DFT) calculations revealed that one Pd(0) center coordinates the departing iodine atom in a halogen bond-like fashion, while another one inserts in the C–I bond (pictured). This conceptually novel cooperative mechanism could also happen in other systems. According to the researchers, this insight may be exploited to design metal-based catalysts for the activation of less reactive carbon-halogen bonds.
- Evidence for a Cooperative Mechanism Involving Two Palladium(0) Centers in the Oxidative Addition of Iodoarenes,
Luca Alessandro Perego, Pierre-Adrien Payard, Baptiste Haddou, Ilaria Ciofini, Laurence Grimaud,
Chem. Eur. J. 2018.
https://doi.org/10.1002/chem.201704899