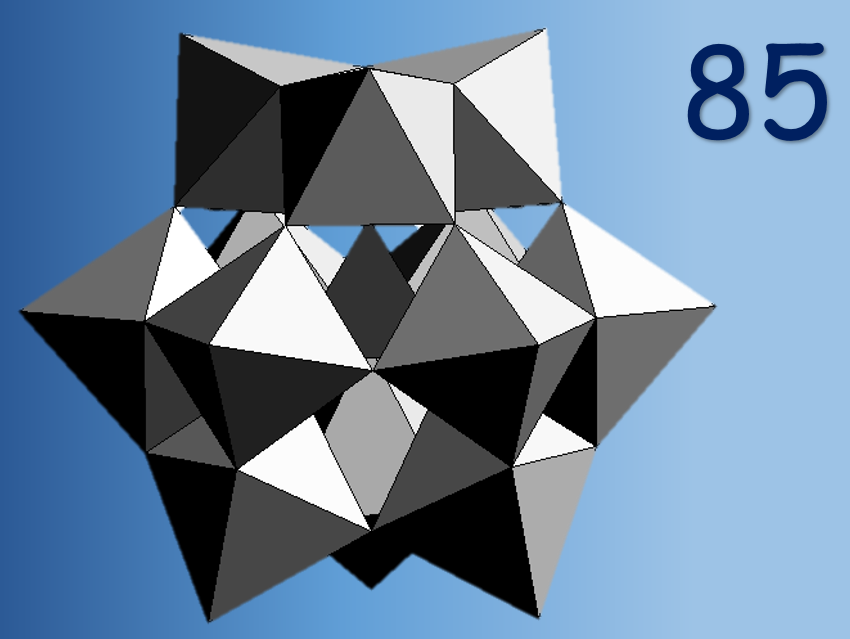
2018 marks the exciting 85th birthday celebration of the “Keggin structure”, which itself is at the center of polyoxometalate (POM) chemistry! The first polyoxoanions were reported almost two centuries ago by Berzelius (1826), but J. F. Keggin could only characterize them structurally in 1933 [1]. He solved the structure of the free acid H3PW12O40 using powder X-ray diffraction, a highly symmetrical metal-oxo anion with tetrahedral symmetry (see Fig. 1). The availability of X-ray diffraction techniques paved the way for the discovery of a large number of novel POMs with different shapes and sizes. Keggin is our POM pioneer!
Similar to the common building block in organic chemistry, the methylene group “–CH2–”, octahedral MO6 (M = Mo, W, V, Nb, Ta) units are the key building blocks of POMs. Such building blocks connect to each other (via corners or edges) upon acidification of aqueous solutions to form many different types of structures (e.g., [V10O28]6–, [Mo7O24]6–, [PW12O40]3–) dictated by reaction conditions such as pH, temperature, concentration and ratio of reagents, ionic strength, and counter-cations.
The beauty of POM chemistry lies in its ability to resemble the “Lego block” game on the microscopic level, for example, by removal of one or more MO6 octahedra from the Keggin ion. The vacancies allow reactions with all kinds of oxophilic electrophiles, leading to novel structures with novel properties. This again emphasizes the relevance of the Keggin structure for its reactive derivatives.
Figure 1. The Keggin ion. WO6 octahedra (red) and heteroatom P (blue).
Another leading figure in the global POM community is M. T. Pope, the author of “Heteropoly and Isopoly Oxometalates”, which remains the bible of POM chemistry (5282+ citations since 1983) [2]. This comprehensive work focuses mainly on synthetic and structural principles, properties, and applications of POMs. Another highlight is the 1991 review article by Pope and A. Müller, that adds diverse dimensions to the field (3215 citations+) [3].
Also, the number of POM-related patents has been growing exponentially during the last decades. The reason for this large interest in these discrete anionic POMs is due to their attractive applications … vide infra … First, let us look at some structural developments that have emerged from the Keggin structure.
Two Structural Highlights
Besides the Keggin ion [XM12O40]n−, several other seminal POM structures are known nowadays, such as Anderson-Evans [XMo6O24]n−, Lindqvist [M6O19]n−, Wells-Dawson [X2M18O62]n−, Pope-Jeannin-Preyssler [NaP5W30O110]14−, and Strandberg [P2Mo5O23]6−. In recent years, both the molybdenum and palladium chemistries have revitalized the POM field, respectively with the following pioneering works that led to unexpected structures with extraordinary physicochemical properties.
(1) The molybdenum-blue chemistry attracted global attention in 1995 with the structural discovery of the spectacular wheel-shaped polyoxomolybdate (4 nm diameter) comprising 154 molybdenum atoms, [Mo154(NO)14O448H14(H2O)70]28−, by A. Müller [4, 5] (see Fig. 2). Since then his research group in Bielefeld, Germany, has systematically extended this work with the discovery of even larger wheels (also known as “Bielefeld wheels” or “Müllerenes”) with 176 and 248 molybdenum atoms [6], and finally even a hedgehog-shaped structure with 368 Mo atoms. As Professor Müller says: “With these materials, we are bringing inorganic chemistry to life”.
(2) The palladium chemistry also witnessed an unexpected, but equally exciting moment in 2008 with the discovery of the first discrete, molecular polyoxopalladate(II) (POP) by the Kortz group [7]. The Kortz ion, [Pd13O8(AsO4)8H6]8–, has a cuboid-shape (1 nm edge-length) and comprises 13 Pd2+ ions in square-planar coordination and eight arsenate (AsO43–) capping groups (see Fig. 2). Since then, mainly the Kortz group has advanced this field with the synthesis of >60 polypalladates of different shape, size, and composition, predominantly based on the {MPd12L8} nanocube and the {MPd15L10} nanostar, by substituting the central metal ion M or the external capping group L [8].
In the last ten years, it has become evident that an entire family of POPs exists and, hence, it is necessary to broaden the classical definition of POM chemistry to include polyoxo-noble-metalates.
Figure 2. (left) The Müllerene structure [4]: Mo (blue), O (red), N (green). (right) The Kortz ion [7]: Pd (blue), As (green), O (red)
Some Featured Applications
POMs have remarkable properties such as oxygen-rich surface, controllable size, composition, charge density, redox potential, acid strength, high thermal stability in the solid-state, solubility in polar/non-polar solvents, and electron/proton storage capacities. The versatility of POMs and their unique ability to be tailor-made are the basis for academic and industrial attention, especially in catalysis and medicine (antibacterial, antiviral, and anti-retroviral activity). Other fields such as material sciences, magnetism, photochemistry, and electrocatalysis were also influenced by POMs. While some POMs have made it to commercial applications in the chemical industry, fundamental and explorative research is still going strong. Some research areas are highlighted below.
Biomedicine
Here are two stories featuring the use of POMs in biomedicine: one leading to human tragedy and the other to the Nobel Prize.
(1) In 1984, France has used the POM abbreviated as {Sb9W21} (known as HPA-23) as an HIV treatment for its assumed antiretroviral activity. Serious side effects were observed as no scientific, controlled trials were previously performed. The American actor Rock Hudson was a victim of this treatment. Clinical studies have later shown that there was no observed improvement in patients using this treatment [9].
(2) In 2009, Ada E. Yonath, Venkatraman Ramakrishnan, and Thomas A Steitz were awarded the Chemistry Nobel Prize for their studies on the structure and function of the ribosome. Did POMs play a role? Indeed.
Adding a minute amount of POM, in particular polyoxotungstates such as [P2W18O62]6–, to the ribosome solution before crystallization did the trick. The quality of the resulting ribosome crystal was much better and the X-ray diffraction (XRD) structure could be solved much more easily, due to the presence of heavy tungsten atoms in the “sea” of carbon atoms from the biomolecule. Overall, XRD structures were obtained with orders of magnitude improved resolution [10]. It is evident that POMs played a small, but significant role in this Nobel-worthy discovery.
Supported POMs as Heterogeneous Catalysts
This is an exciting application for POMs for its academic and industrial importance. Supports could directly influence the catalyst’s activity and selectivity, which makes the immobilization step of POMs a critical catalyst design criterion (support type, porosity, thermal stability, etc.). Various supports were adopted with POMs including, but not limited to, mesoporous silica-based materials, metal-organic frameworks (MOFs), mesoporous polymers, metal oxides, zeolites [11].
Here we highlight the use of MOFs as a support, a class of materials composed of an ordered periodic network of molecular components (metal ions or clusters) interconnected in three dimensions by organic ligands (linkers). This leads to framework structures with cavities or pores [12–14].
Within the last two decades, POM-based MOFs offered new horizons for heterogeneous catalysis: Encapsulating classical POMs into MOF cavities have been mainly investigated by using the Keggin, Wells-Dawson, and Lindqvist-type ions [15–17]. For example, G. Férey et al. incorporated the mono-lacunary Keggin ion PW11O397– into MIL-101, a highly porous MOF [13]. Later, titanium- and cobalt-substituted Keggin ions were also bound to MIL-101 and used as heterogeneous catalysts for oxidation reactions [18, 19]. Many other examples are present in the literature [20–24]. This year, polyoxopalladate and MOF chemistries were merged leading to the first example of a porous and stable POP-based MOF (JUB-1). It shows attractive sorption properties and catalytic activity for Suzuki-Miyaura coupling reactions [25].
Functional Components in Nanotechnological Devices
The ability of POMs to accept multiple electrons and undergo rapid reversible reductions with minimal structural changes renders them interesting for building functional materials and devices. A review article in 2008 by D. G. Kurth, summarizes the recent developments to fabricate and characterize thin films of well-defined compositions and dimensions with supramolecular modules, such as POMs [26]. Kurth highlights the many different examples of POMs that were successfully incorporated in multilayers demonstrating their potential through proof-of-principle experiments.
For example, a multilayer composed of the europium-substituted Pope-Jeannin-Preyssler ion (EuP5W30O11012–) as one of its components showed optical memory. This is a promising feature for electrochromic devices: after reduction, the layer remained in its reduced, colored state even after cutting the potential source. This means that this device has advantages of low operation voltage and low power consumption.
In 2014, L. Cronin’s group designed and fabricated memory devices mainly based on Wells-Dawson type POMs (X2M18O62n–) [27]. Memory cells are the basis of many electronics (USB sticks, smartphones, etc.) and there is a need apparently to decrease the size of future componentes for electronic devices. The potential of information storage is tuned by the number of electrons exchanged, which makes POMs highly-suitable candidates, as their redox activity can be structurally and compositionally tuned, and hence, the bits of information that can be stored. In the same year, the groups of Coronado, Proust, and Guldi showed the potential of pyrene-appended Wells-Dawsons type POMs self-assembled with single-walled carbon nanotubes in photoactive molecular devices [28].
An Invitation to Meet the POM Community
As you can see, the spectrum of POM applications is very wide making this field extremely interdisciplinary. Almost every year, POM scientists from around the world meet to discuss latest research findings and initiate collaborations. In recent years, such international POM meetings have taken place for example in Bremen (2009), Jerusalem (2010), Honolulu (2010), Lanzarote (2012), Tenerife (2013), Maffliers (2014), Honolulu (2015), Newcastle upon Tyne (2016), and Changchun (2017).
The next POM meeting will be this summer in Sendai, Japan (30 July–4 August 2018) in the context of the ICCC conference (session 1, session 2), followed by a POM workshop in Tokyo, Japan (5–8 August 2018). These events are mainly organized by the Japanese POM colleagues Tomoji Ozeki, Masahiro Sadakane, and Sayaka Uchida. The European Journal of Inorganic Chemistry (EurJIC) will publish a special issue on the ICCC conference in December.
References
[1] J. F. Keggin, Structure of the Molecule of 12-Phosphotungstic Acid, Nature 1933, 131, 908. https://doi.org/10.1038/131908b0
[2] M. T. Pope, Heteropoly and Isopoly Oxometalates, Springer-Verlag, Berlin, 1983.
[3] M. T. Pope, A. Müller, Polyoxometalate Chemistry: An Old Field with New Dimensions in Several Disciplines, Angew. Chem. Int. Ed. 1991, 30, 34–48. https://doi.org/10.1002/anie.199100341
[4] A. Müller, E. Krickemeyer, J. Meyer, H. Bogge, F. Peters, W. Plass, E. Diemann, S. Dillinger, F. Nonnebruch, M. Randerath, C. Menke, [Mo154(NO)14O420(OH)28(H2O)70](25 ± 5)−: A Water-Soluble Big Wheel with More than 700 Atoms and a Relative Molecular Mass of About 24000, Angew. Chem. Int. Ed. 1995, 34, 2122–2124. https://doi.org/10.1002/anie.199521221
[5] A. Müller, C. Serain, Soluble Molybdenum Blues – “des Pudels Kern”, Acc. Chem. Res. 2000, 33(1), 2–10. https://doi.org/10.1021/ar9601510
[6] A. Müller, Syed Q. N. Shah, H. Bögge, M. Schmidtmann, Molecular growth from a Mo176 to a Mo248 cluster, Nature 1999, 397, 48–50. https://doi.org/10.1038/16215
[7] E. V. Chubarova, M. H. Dickman, B. Keita, L. Nadjo, F. Miserque, M. Mifsud, I. W. C. E. Arends, U. Kortz, Self-Assembly of a Heteropolyoxopalladate Nanocube: [PdII13AsV8O34(OH)6]8−, Angew. Chem. Int. Ed. 2008, 47(49), 9542–9546. https://doi.org/10.1002/anie.200803527
[8] N. V. Izarova, M. T. Pope, U. Kortz, Noble Metals in Polyoxometalates, Angew. Chem. Int. Ed. 2012, 51, 9492–9510. https://doi.org/10.1002/anie.201202750
[9] B. L. Moskovitz, Clinical trial of tolerance of HPA-23 in patients with acquired immune deficiency syndrome, Antimicrob. Agents Chemother. 1988, 32(9), 1300–1303. https://doi.org/10.1128/AAC.32.9.1300
[10] A. Tocilj, F. Schlünzen, D. Janell, M. Glühmann, H. A. S. Hansen, J. Harms, A. Bashan, H. Bartels, I. Agmon, F. Franceschi, A. Yonath, The small ribosomal subunit from Thermus thermophilus at 4.5 Å resolution: Pattern fittings and the identification of a functional site, Proc. Natl. Acad. Sci. 1999, 96(25), 14252–14257. https://doi.org/10.1073/pnas.96.25.14252
[11] Y. Zhou, G. Chen, Z. Long, J. Wang, Recent advances in polyoxometalate-based heterogeneous catalytic materials for liquid-phase organic transformations, RSC Adv. 2014, 4, 42092–42113. https://doi.org/10.1039/C4RA05175K
[12] H. Li, M. Eddaoudi, M. O’Keeffe, O. M. Yaghi, Design and synthesis of an exceptionally stable and highly porous metal-organic framework, Nature 1999, 402, 276–279. https://doi.org/10.1038/46248
[13] G. Férey, C. M. Draznieks, C. Serre, F. Millange, J. Dutour, S. Surble, I. Margiolaki, A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area, Science 2005, 309(5743), 2040–2042. https://doi.org/10.1126/science.1116275
[14] Special Issue on Metal-Organic Frameworks, Chem. Rev. 2012, 112, 673–1268. https://doi.org/10.1021/cr300014x
[15] J. Song, Z. Luo, D. K. Britt, H. Furukawa, O. M. Yaghi, K. I. Hardcastle, C. L. Hill, A Multiunit Catalyst with Synergistic Stability and Reactivity: A Polyoxometalate–Metal Organic Framework for Aerobic Decontamination, J. Am. Chem. Soc. 2011, 133(42), 16839–16846. https://doi.org/10.1021/ja203695h
[16] D.-Y. Du, J.-S. Qin, S.-L. Li, Z.-M. Su, Y.-Q. Lan, Recent advances in porous polyoxometalate-based metal–organic framework materials, Chem. Soc. Rev. 2014, 43, 4615–4632. https://doi.org/10.1039/C3CS60404G
[17] Z.-M. Zhang, T. Zhang, C. Wang, Z. Lin, L.-S. Long, W. Lin, J. Am. Chem. Soc. 2015, 137, 3197–3200. identisch zu 21
[18] N. V. Maksimchuk, M. N. Timofeeva, M. S. Melgunov, A. N. Shmakov, Y. A. Chesalov, D. N. Dybtsev, V. P. Fedin, O. A. Kholdeeva, Heterogeneous selective oxidation catalysts based on coordination polymer MIL-101 and transition metal-substituted polyoxometalates, J. Catal. 2008, 257(2), 315–323. https://doi.org/10.1016/j.jcat.2008.05.014
[19] N. V. Maksimchuk, K. A. Kovalenko, S. S. Arzumanov, Y. A. Chesalov, M. S. Melgunov, A. G. Stepanov, V. P. Fedin, O. A. Kholdeeva, Hybrid Polyoxotungstate/MIL-101 Materials: Synthesis, Characterization, and Catalysis of H2O2-Based Alkene Epoxidation, Inorg. Chem. 2010, 49(6), 2920–2930. https://doi.org/10.1021/ic902459f
[20] J. Song, Z. Luo, D. K. Britt, H. Furukawa, O. M. Yaghi, K. I. Hardcastle, C. L. Hill, A Multiunit Catalyst with Synergistic Stability and Reactivity: A Polyoxometalate–Metal Organic Framework for Aerobic Decontamination, J. Am. Chem. Soc. 2011, 133(42), 16839–16846. https://doi.org/10.1021/ja203695h
[21] Z.-M. Zhang, T. Zhang, C. Wang, Z. Lin, L.-S. Long, W. Lin, Photosensitizing Metal–Organic Framework Enabling Visible-Light-Driven Proton Reduction by a Wells–Dawson-Type Polyoxometalate, J. Am. Chem. Soc. 2015, 137(9), 3197–3200. https://doi.org/10.1021/jacs.5b00075
[22] W. Salomon, Y. Lan, E. Riviére, S. Yang, C. Roch-Marchal, A. Dolbecq, C. Simonnet-Jégat, N. Steunou, N. Leclerc-Laronze, L. Ruhlmann, T. Mallah, W. Wernsdorfer, P. Mialane, Single-Molecule Magnet Behavior of Individual Polyoxometalate Molecules Incorporated within Biopolymer or Metal–Organic Framework Matrices, Chem. Eur. J. 2016, 22, 6564–6574. https://doi.org/10.1002/chem.201600202
[23] B. Nohra, H. El Moll, L. M. Rodriguez Albelo, P. Mialane, J. Marrot, C. Mellot-Draznieks, M. O’Keeffe, R. Ngo Biboum, J. Lemaire, B. Keita, L. Nadjo, A. Dolbecq, Polyoxometalate-Based Metal Organic Frameworks (POMOFs): Structural Trends, Energetics, and High Electrocatalytic Efficiency for Hydrogen Evolution Reaction, J. Am. Chem. Soc. 2011, 133(34), 13363–13374. https://doi.org/10.1021/ja201165c
[24] S. G. Mitchell, C. Streb, H. N. Miras, T. Boyd, D.-L. Long, L. Cronin, Face-directed self-assembly of an electronically active Archimedean polyoxometalate architecture, Nat. Chem. 2010, 2, 308–312. https://doi.org/10.1038/nchem.581
[25] S. Bhattacharya, W. W. Ayass, D. H. Taffa, A. Schneemann, A. L. Semrau, S. Wannapaiboon, P. J. Altmann, A. Pöthig, T. Nisar, T. Balster, V. Wagner, R. A. Fischer, M. Wark, U. Kortz, submitted 2018.
[26] D. G. Kurth, Metallo-supramolecular modules as a paradigm for materials science, Sci. Technol. Adv. Mater. 2008, 9, 1–25. https://doi.org/10.1088/1468-6996/9/1/014103
[27] C. Busche, L. Vilà-Nadal, J. Yan, H. N. Miras, D.-L. Long, V. P. Georgiev, A. Asenov, R. H. Pedersen, N. Gadegaard, M. M. Mirza, D. J. Paul, J. M. Poblet, L. Cronin, Design and fabrication of memory devices based on nanoscale polyoxometalate clusters, Nature 2014, 515, 545–549. https://doi.org/10.1038/nature13951
[28] C. Bosch-Navarro, B. Matt, G. Izzet, C. Romero-Nieto, K. Dirian, A. Raya, S. I. Molina, A. Proust, D. M. Guldi, C. Marti-Gastaldo, E. Coronado, Charge transfer interactions in self-assembled single walled carbon nanotubes/Dawson–Wells polyoxometalate hybrids, Chem. Sci. 2014, 5, 4346–4354. https://doi.org/ 10.1039/C4SC01335B
Also of Interest
- Polyoxometalate Octagenarians,
Interview with polyoxometalate chemists about the fascination and future trends of their research field
Karen Hindson, Vera Koester, Bernt Krebs, André Tézé, Toshihiro Yamase,
ChemViews Mag. 2018.
https://doi.org/10.1002/chemv.201800007 - Special Issue:Celebrating Polyoxometalates,
Eur. J. Inorg. Chem. 2019.