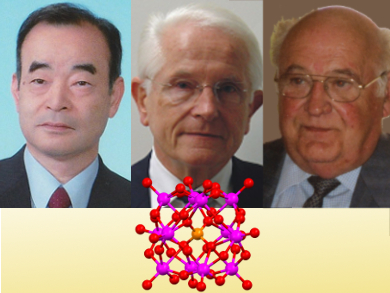
2018 is an important year for polyoxometalates, it marks the 85th anniversary of J. F. Keggin’s Nature paper reporting the Keggin structure [1, 2]. The Keggin ion was the first structure of a polyoxometalate (POM) that was determined.
Dr. Karen Hindson and Dr. Vera Koester spoke for ChemViews Magazine with polyoxometalate chemists, who also have anniversaries in this year: Professor André Tézé (28.1.1938), France, and Professor Bernt Krebs (26.11.1938), Germany. The youngest of the group, Professor Toshihiro Yamase (1.1.1943) Japan, is not an octogenarian, but was born ten years after the publication of the Keggin structure. Two further octagenarians mentioned in the interviews, Professor Michael T. Pope (14.4.1933), UK, and Professor Achim Müller (14.2.1938), Germany, were not available for comment at this time.
What got you interested in polyoxometalate chemistry?
André Tézé: I had decided to become a teacher. In 1960, I was asked to perform a training period in a research laboratory. At the time Professor Souchay was a young and much-appreciated teacher of thermodynamics at the Sorbonne. In his laboratory, I discovered the world of polyoxometalate chemistry.
Bernt Krebs: When I did my chemistry studies at Oskar Glemser´s institute in Göttingen, polyoxometalates was one of his research fields. As a person who was much interested in architecture – I almost had selected architecture instead of chemistry as my field of university studies – I was most impressed from the beginning by the fantastic structural beauty and chemical variety of the POMs, starting with the iconic Keggin ion.
Consequently, I invested much effort in our first major project, namely the crystallization and full crystal structure of the 36-isopolymolybdate which was at the time the largest distinct transition metal polymetalate known. At that time, in the sixties of the last century, the X-ray structure determination of such a rather large system was still a challenge for a structural chemist.
Toshihiro Yamase: I came from the study of metal oxides and was fascinated by polyoxometalates as a good models for understanding a variety of physicochemical behaviors of such metal-oxide materials.
How has polyoxometalate research developed over the years?
André Tézé: In the 1960s, there were only a few groups worldwide working on POM chemistry. In addition to Paris the two other main groups were in Washington DC, USA (Professor L. C. W. Baker) and in Moscow, Russia (Professor V. I. Spitsyn). Several students of these labs managed to increase the visibility of this research topic over the years and attracted new researchers to work in this area.
Initial studies of POM chemistry employed mainly electrochemical methods and electronic spectroscopies. The use of single crystal X-ray diffraction and multinuclear NMR have much enlarged the field of research, but synthesis in a simple beaker remains the basis of this exciting chemistry. Nowadays, the number of labs worldwide and the diversity of POM research truly astonish me.
Bernt Krebs: Polyoxometalate chemistry has experienced a continuous development during the last decades. It’s well-established universal significance from structural and topological theory to analytical applications, to the most important catalytic power of POMs, to possible biological and medical applications, and, lately, to supramolecular functional entities have kept permanent interest and fascination on polyoxometalates alive.
What have been the most exciting developments?
André Tézé: In my own career, the research subject of my “doctorat ès sciences” was on isomerism of heteropolytungstates. I synthesized several tungstosilicates and every time I discovered a novel compound and determined the structure of a new isomer was a great moment.
Bernt Krebs: The most exciting developments in polyoxometalate chemistry for me are the synthesis of the remarkable macro-POMs including the Keplerates, the big wheels and the functional container systems by Achim Müller, and, in addition, maybe novel classes of organosubstituted species which might pave new ways toward selective new heterogeneous or homogeneous catalysts.
Toshihiro Yamase: For me, supramolecular chemistry starting at {Mo14}- based Mo-blue, the molecular magnetism of the V312+-triangle, the Cu48+-tetragon and the Cu612+-hexagon, and the anti-tumor, anti-viral and anti-bacterial activities, as well as energy conversions were most exciting.
André Tézé: When the antiviral properties of POMs were tested was a very exciting and pleasing period. It was a pleasure to communicate and exchange ideas with medicinal scientists. Many young people do not know anymore that one POM (Sb9W21) was actually approved in France for clinical use. For instance, the American actor Rock Hudson was flown to Paris in 1985 for AIDS treatment.
What are the interesting aspects under study now?
André Tézé: The chemistry of POMs has become so broad ranging from synthesis and structural characterization to catalysis, energy, and biology and I consider all of them potentially interesting. It should be remembered that POMs have contributed to the Chemistry Nobel Prize in 2009 – the structural characterization of ribosomes – as cocrystallization agents.
Bernt Krebs: There are many interesting aspects under study now. Indeed, a very valuable application of POMs remains continuously their successful application as heavy-atom reagents to solve the phase problem in protein and nucleic acid structure determination.
I think challenging objectives of current POM research are also theoretical studies of bonding and reactive properties with DFT methods and broad studies on catalytic properties, especially within confined spaces of macro-POMs.
Toshihiro Yamase: I think proton-coupled electron transfer in the lattice and a realization of a commercial possibility of an electroluminescence-paper device for lighting applications and large-size displays are of importance. They are of importance to understand biological electron-transfer processes and to manufacture large-area, flexible, high-performance displays, respectively.
How do you think the field will develop in the future?
Bernt Krebs: One aspect will be the development of more straightforward partially automated synthetic procedures for extending the synthetic possibilities. Furthermore, aspects mentioned already, namely supramolecular functional systems, reactive POM systems in general, most importantly the extension of the possibilities of selective catalysis, e.g., adding more hetero metals, and confined reaction systems should be subjects of future research.
I strongly propose to give more attention to another aspect which has been neglected too much lately, namely research on biological, biomedical, and therapeutic effects of POMs. There must be most interesting possibilities of positive effects.
Moreover, China`s growing role also in POM chemistry should not be forgotten. Already today Chinese research groups are contributing largely to the scientific output within POM chemistry.
Toshihiro Yamase: In addition, Solar energy conversion and nuclear chemistry are future trends.
André Tézé: I think the field has a very bright future. The field is so dynamic that I am ready for any surprises
Who or what has inspired you most in chemistry and during your career?
André Tézé: Many people! J. F. Keggin and his POM structure is one of them. I have always been fascinated by POM structural chemistry which is mainly based on shared octahedra, allowing the formation of large discrete ions. I also think that Michael Pope has done inspiring work on POMs for decades. Nowadays, due to the significant improvement in crystallography – allowing the measurement of new POM structures in a few hours – and sophisticated visualization software, many young scientists have perhaps forgotten to appreciate the true beauty of POM structures. There is a famous saying: “A thing of beauty is a joy forever“.
Bernt Krebs: Some of my inspirations are the miracles and challenges of chemical catalysis, amnd the power of physical and analytical methods, especially structural analysis by diffraction methods, showing the amazing architecture of molecules. Then also for me the fantastic power of metals in living systems developed during evolution and the unexpected findings such as noble gas compounds.
And, last but not least, the fascination to see how every scientist stands on the shoulder of others.
What motivates you, Professor Krebs?
Bernt Krebs: The responsibility of me as a scientist to our society. To master the problems connected with globalization and to solve the world energy and climate problems, we are facing the challenge to find new insights and new products as a chemist. We must find solutions for the relationship between rich and poor countries, which underlines the necessity to convince society and especially the young generation and our students that experimentally founded scientific insight should be the basis of our thinking rather than irrational and ideological action.
What are your plans for the future, Professor Yamase?
Toshihiro Yamase: There remain unpublished data about polyoxometalate chemistry which I would compile after additional acquaintance, and i am working towards realizing commercial possibilities of a few discoveries
What other interests do you have?
Toshihiro Yamase: I enjoy walking and bird watching.
André Tézé: I like genealogy, reading, and swimming. As often as possible I spend time with my grandchildren and follow the POM chemistry of my friends and former students and colleagues.
Bernt Krebs: I have an active interest and participation in art, architecture, and play music. I collect, e.g., rare pieces of art and rare stamps. And I also keep fit with some sports.
Thank you very much for the interview.
 |
Bernt Krebs studied chemistry at the University of Göttingen, Germany, and received his Ph.D. there for work on trithiocarbonic acid and its chemistry under the supervision of Gerhard Gattow. After postdoctoral work in the laboratory of Walter Hamilton at Brookhaven National Laboratory, Upton, NY, USA, from 1965–1966, he completed his habilitation at Göttingen University in 1969. In 1971, he became associate professor at the University of Kiel, Germany, in 1974, full professor at the newly founded University of Bielefeld, Germany, and in 1976, full professor at the University of Münster, Germany. Since 2003, he is Professor Emeritus. His primary research interests included chemical and structural studies on thio and seleno compounds, chalcogen halides, chalcogenide solid-state compounds, transition metal oxygen compounds, cis-platin-A analogues, and metalloproteins as well as model complexes for these metalloproteins. |
 |
André Tézé studied sciences at the École Normale Supérieure de Cachan, and chemistry at the Sorbonne, Paris, both France. After teaching chemistry in an engineering school in Rouen, France, he was appointed professor at the University Pierre-et-Marie-Curie, Paris, France, in 1969. In 1990, he moved to the newly founded University of Versailles, France. He is Professor Emeritus since 2000. |
 |
Toshihiro Yamase received a B.S. in 1965 from Nagoya Institute of Technology, Japan, and obtained a Ph.D. in 1970 for his studies of the sensitized photolysis of dithiocarbamates from Tokyo Institute of Technology, Japan, with Eiichi Inoue. He became a research associate with Tsuneo Ikawa in 1971. After postdoctoral work on dye sensitization on the semiconductor electrode at Fritz-Haber-Institut der Max-Planck-Gesellschaft, Germany, with Heinz Gerischer from 1976 to 1977, he joined Tokyo Institute of Technology again in 1977 and became full Professor in 1989. His research efforts have been in the structure, photochemistry, and biological activity of polyoxometalates. His research interest is molecular devices based on polyoxometalates. |
Selected Publications Krebs
- B. Krebs, I. Paulat-Böschen, The Structure of the Potassium Isopolymolybdate K8[Mo36O112(H2O)16].nH2O (n = 36..40), Acta Cryst. 1982, B38, 1710–1718. https://doi.org/10.1107/S0567740882007018
- M. Bösing, I. Loose, H. Pohlmann, B. Krebs, New Strategies for the Generation of Large Heteropolymetalate Clusters: The beta-B-SbW9-Fragment as a Multifunctional Unit, Chem. Eur. J. 1997, 3, 1232–1237. https://doi.org/10.1002/chem.19970030810
- M. Bösing, A. Nöh, I. Loose, B. Krebs, Highly Efficient Catalysts in Directed Oxygen-Transfer Processes: Synthesis, Structures of Novel Manganese-Containing Heteropolyanions and Applications in Regioselective Epoxidation of Dienes with Hydrogen Peroxide, J. Am. Chem. Soc. 1998, 120, 7252–7259. https://doi.org/10.1021/ja974281v
- D. Volkmer, B. Bredenkötter, J. Tellenbröker, P. Kögerler, D.G. Kurth, P. Lehmann, H. Schnablegger, D. Schwahn, M. Piepenbrink, B. Krebs, Structure and Properties of the Dendron-Encapsulated Polyoxometalate (C52H60NO12)12[(Mn(H2O))3(SbW9O33)2], A First Generation Dendrozyme, J. Am. Chem. Soc. 2002, 124, 10489–10496. https://doi.org/10.1021/ja017613b
- M. Piepenbrink, M. U. Triller, N. H. J. Gorman, B. Krebs, Bridging the Gap between Polyoxometalates and Classic Coordination Compounds: A Novel Type of Hexavanadate Complex, Angew. Chem. Int. Ed. 2002, 41, 2523–2525. Syntheses and X-ray Characterization of Novel [M4(H2O)2(XW9O34)2]n– (M = FeIII, CuII; X = FeIII, CuII) Polyoxotungstates, J. Cluster Sci. 2002, 13, 369 (see also Z. Naturforsch. 2004, 59B, 980–984). https://doi.org/10.1023/A:1020551016077
- D. Drewes, M. Piepenbrink, B. Krebs, [Ho5(H2O)16(OH)2As6W64O220]25–, A Large Novel Polyoxoanion Containing Trivacant Keggin Fragments, Z. Anorg. Allg. Chem. 2006, 632, 534–536. https://doi.org/10.1002/zaac.200500482
Selected Publications André Tézé
- C. Jasmin, J.-C. Chermann, G. Hervé, A. Tézé, P. Souchay, C. Boy-Loustau, N. Raybaud, F. Sinoussi, M. Raynaud, In Vivo Inhibition of Murine Leukemia and Sarcoma Viruses by the Heteropolyanion 5-Tungsto-2- antimonate, J. Natl. Cancer. Inst. 1974, 53, 469. https://doi.org/10.1093/jnci/53.2.469
- N. Larnicol, V. Augery, C. Lebousse-Kerdiles, V. Degiorgis, J. C. Chermann, A. Tézé, C. Jasmin, In vivo Effect of a New Mineral Condensed Ion (HPA 39) on Murine Friend Leukemia, J. Gen. Virol. 1981, 55, 17. https://doi.org/10.1099/0022-1317-55-1-17
- Roland Contant, André Tézé, A New Crown Heteropolyanion K28Li5H7P8W48O184·92H2O: Synthesis, Structure and Properties, Inorg. Chem. 1985, 24, 4610–4614. https://doi.org/10.1021/ic00220a036
- J. Canny, R. Thouvenot, A. Tézé, G. Hervé, M. Leparulo-Loftus, M. T. Pope, Disubstituted Tungstosilicates. 2. γ and β Isomers of SiW10V2O406– : Synthesis and Structure determination by 183W, 51V and 29Si NMR Spectroscopy, Inorg. Chem. 1991, 30, 976. https://doi.org/10.1021/ic00005a020
- A. Tézé, J. Canny, L. Gurban, R. Thouvenot, G. Hervé, Synthesis, Structural Characterization and Oxidation-Reduction Behavior of the γ-isomer of the Dodecatungstosilicate anion, Inorg. Chem. 1996, 35, 1001–1005. https://doi.org/10.1021/ic951004l
- U. Kortz, Y. Jeannin, A. Tézé, G. Hervé, S. Isber, A Novel Dimeric Ni-Substituted β-Keggin Silicotungstate: Structure and Magnetic Properties of K12[{β-SiNi2W10O36(OH)2(H2O)}2]·20H2O, Inorg. Chem. 1999, 38(16), 3670–3675. https://doi.org/10.1021/ic990008d
- A. Tézé, E. Cadot, V. Béreau, G. Hervé, About the Keggin Isomers: Crystal Structure of [N(C4H9)4]4-γ-[SiW12O40], the γ-isomer of the Keggin Ion. Synthesis and 183W NMR. Characterization of the Mixed γ-[SiMo2W10O40]n- (n = 4 or 6), Inorg. Chem. 2001, 40(9), 2000–2004. https://doi.org/10.1021/ic000864l
- A. Tézé, C. Marchal-Roch, H. So, M. Fournier, G. Hervé, Synthesis, X-ray crystal structure, and EPR study of [Na(H2O)2]2[VO(H2O)5] [SiW12O40] 4H2O, Solid State Sci. 2001, 3(3), 329–338. https://doi.org/10.1016/S1293-2558(00)01136-5
Selected Publications Toshihiro Yamase
- T. Yamase, M. Suga, Photochemical Studies of Alkylammonium Molybdates. Part 8. Location of Protons Interacting with Paramagnetic Electron in a Single Crystal of Photoirradiated [NH3Pri]6[Mo8O26(OH)2]・2H2O, J. Chem. Soc. Dalton Trans. 1989, 661–669. https://doi.org/ 10.1039/DT9890000661
- T. Yamase, Photochemical Studies of Alkylammonium Molybdates. Part 9. Structure of Diamagnetic Blue Species Involved in the Photoredox Reaction of [Mo7O24]6–, J. Chem. Soc. Dalton Trans. 1991, 3055–3063. https://doi.org/10.1039/DT9910003055
- T. Yamase, K. Fukaya, E. Ishikawa, H. Nojiri, T. Taniguchi, T. Atake, Spin-frustrated (VO3)6+-Triangle-sandwiching Octadecatungstates as a New Class of Molecular Magnets, Inorg. Chem. 2004, 43, 8150–8157. https://doi.org/10.1021/ic049669n
- T. Yamase, S. Kumagai, P. Prokop, E. Ishikawa, A.-R. Tomsa, {Mo96La8} Eggshell Ring and Self-Assembly to {Mo132} Keplerate through Mo-blue Intermediate, Involved in UV-Photolysis of [Mo7O24]6–/Carboxylic Acid System at pH 4, Inorg. Chem. 2010, 49, 9426–9437. https://doi.org/10.1021/ic101027d
- T. Yamase, H. Ishikawa, H. Abe, K. Fukaya, H. Nojiri, H. Takeuchi, Molecular Magnetism of M6-Hexagon Ring in D3d-Symmetric [(MCl)6(XW9O33)2]12– (M = CuII and MnII, X = SbIII and AsIII), Inorg. Chem. 2012, 51, 4606–4619. https://doi.org/10.1021/ic202513q
References
- J. F. Keggin, Structure of the Molecule of 12-Phosphotungstic Acid, Nature 1933, 131, 908. https://doi.org/10.1038/131908b0
- J. F. Keggin, The structure and formula of 12-phosphotungstic acid, Proc. Roy. Chem. Soc. A 1934, 144, 75. https://doi.org/10.1098/rspa.1934.0035
Also of Interest
- Remember the Keggin Ion?,
Wassim W. Ayass and Ulrich Kortz,
ChemViews Mag. 2018.
https://doi.org/10.1002/chemv.201800009 - Special Issue:Celebrating Polyoxometalates,
Eur. J. Inorg. Chem. 2019.