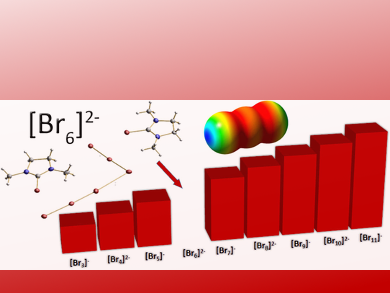
Underexplored Polybromides
Polyhalogenides are promising materials for electrochemical devices and batteries. Especially the polybromides are appealing because of their potential to store elemental bromine. This element, besides its application in chemical synthesis, has great potential in battery technology. The more it is surprising that the chemistry of the polybromides still appears underexplored. A German research team has now identified and structurally characterized the last polybromide in a series of polyanions and found an unusual hockey-stick-type six-atom structure.
The search for redox-active compounds for electrochemical devices has brought the polyhalides back into focus. Polyhalides can “wrap” or mask the redox-active element—the halogen—in an anionic network of high conductivity. For example, the insoluble iodine can be made soluble in the form of the triiodide anion. The lighter bromine holds great promise as a redox-active component for electrochemistry, for example, in the redox flow battery, for which commercial applications already exist. However, its handling is problematic because elemental bromine is highly toxic and corrosive. Polybromides could make the handling safer, but they have been far less investigated than, for example, the polyiodides. With the hexabromide dianion, Sebastian Riedel and his colleagues at Freie Universität Berlin, Germany, have found the last missing piece in the series of lower polybromides.
Hexabromide Dianion
The team synthesized the polyanion by reacting oxalyl bromide with a urea-derived compound to form bromoimidazolium bromides. By crystallography of the obtained crystals, they discovered that the bromide dianion was made of six bromine atoms in “L” or hockey-stick-type arrangement. This structure was a surprise because calculations on a putative hexabromide dianion favored a T-shape configuration made of two tribromides. The researchers explained the difference by the interaction of the bromides with the two bulky counter ions, which holds the hockey stick arrangement in place and compensates for the additional energy.
The structural data also revealed an asymmetry in the atomic distances and angles between the bromine atoms, and the researchers pointed out that the structure can also be considered as a bromide anion coordinated by a bromine molecule that in turn bridges to a tribromide anion. This view takes into account the general model of polybromide formation as an interaction of the Lewis base bromide with elemental bromine. Thus, the hexabromide dianion appears to have the substructure of two tribromides, one of which is highly asymmetric, coordinated to each other in a hockey-stick-type arrangement.
Closing the Gap
The structure fills a long-existing gap in the series of polybromides from the tribromide to the undecabromide. It also highlights a general aspect of polyhalides: they are coordination structures of halide anions with the dihalogen. Thus, the halogen is “masked” in a polyhalide. Such masking can be helpful if less reactivity and toxicity is desired for practical applications such as in the redox flow battery and solar cells.
Especially the redox flow batteries are experiencing rising (and renewed) interest as scalable and long-living alternatives to lithium-ion batteries. Zinc-bromine redox flow batteries have already been commercialized. They still face huge problems in handling elemental bromine. Polybromides could help.
- Closing the Gap: Structural Evidence for the Missing Hexabromide Dianion [Br6]2–,
Karsten Sonnenberg, Patrick Prçhm, Carsten Müller, Helmut Beckers, Simon Steinhauer, Dieter Lentz, Sebastian Riedel,
Chem. Eur. J. 2018.
https://doi.org/10.1002/chem.201705912