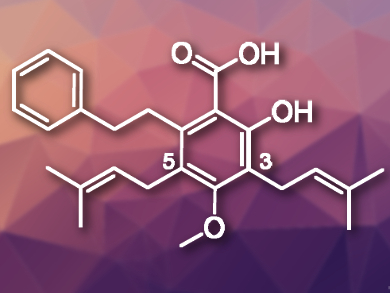
Amorfrutins are a family of natural products with a characteristic planar 2-hydroxybenzoic acid core. Some of these compounds are bioactive. Amorfrutin C (pictured), for example, was isolated from the roots of Glycyrrhiza foetida, a plant related to licorice. This natural product can inhibit human cancer cell proliferation.
Weijia Xie, China Pharmaceutical University, Nanjing, and colleagues have performed the first total syntheses of Amorfrutin C and the related compound pseudo-Amorfrutin A. The team started from commercially available 2,4,6-trihydroxybenzoic acid, which was converted to a protected 2,4-dihydroxybenzoic acid derivative. A prenyl moiety was introduced to this intermediate via a bromination reaction and a subsequent CuCN-meditated alkylation reaction. Using N-bromosuccinimide (NBS) to mediate the bromination led to the monoprenylated pseudo-Amorfrutin A. In contrast, using 1,3-dibromo-5,5-dimethylhydantoin (DBDMH) caused bromination at two positions of the central ring and led to the diprenylated Amorfrutin C.
The synthesis of Amorfrutin C was achieved with an overall yield of 16 % and that of pseudo-Amorfrutin A with a total yield of 29.3 %. According to the researchers, the results could be important for the further design and synthesis of Amorfrutin family derivatives in order to identify drug candidates.
- First total syntheses of Amorfrutin C and pseudo-Amorfrutin A,
Qi Miao, Yunzhi Li, Jinyi Xu, Aijun Lin, Genzoh Tanabe, Osamu Muraoka, Xiaoming Wu, Weijia Xie,
Eur. J. Org. Chem. 2018.
https://doi.org/10.1002/ejoc.201701811