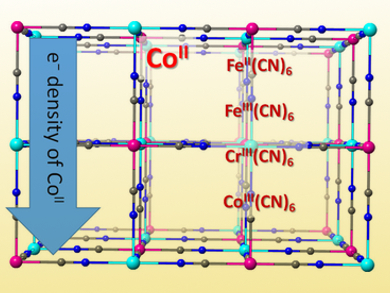
Prussian blue analogues (PBAs) are a family of inorganic polymers with potentially useful electronic and magnetic properties. Yavuz Dede, Gazi University, Ferdi Karadas, Bilkent University, both Ankara, Turkey, and colleagues have investigated the role of PBAs as efficient and robust electrocatalysts for water oxidation using both experimental and computational methods. Electrochemical studies were performed on a series of PBAs, namely Cox[M(CN)6]y (M = Fe2+/3+, Cr3+, and Co3+), to study the effect of neighboring metal ions on the electronic properties of the catalytically active cobalt sites.
A close relationship between the electron density of the electroactive cobalt sites and electrocatalytic activity was found. The catalytic activity could simply be correlated with the shift in cyanide stretching vibration of a PBA in infrared (IR) spectroscopy. This gives indirect information about the electronic properties of the cyanide-based compounds. This correlation, together with computational studies, led to the conclusion that a nucleophilic attack of water to cobalt-oxo species should be the rate-determining step. The results show that the type of the electrocatalytically inactive M(CN)6 fragment in PBAs determines the effectiveness of the water oxidation electrocatalysts.
- Tuning the Electronic Properties of Prussian Blue Analogues for Efficient Water Oxidation Electrocatalysis: Experimental and Computational Studies,
Elif Pınar Alsaç, Emine Ülker, Satya Vijaya Kumar Nune, Yavuz Dede, Ferdi Karadas,
Chem. Eur. J. 2018.
https://doi.org/10.1002/chem.201704933