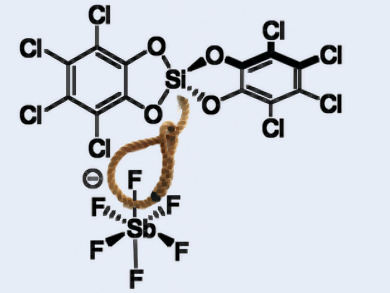
Lewis super acids can be used in bond activation reactions and catalysis. Fluoride ion affinity is a measure of superacidity, and super acids are defined as molecules that can abstract a fluoride ion from SbF6–. While there are strong Lewis acids with a neutral silicon(IV) as the central atom, no neutral silicon Lewis super acid had been found so far.
Lutz Greb and colleagues, University of Heidelberg, Germany, have synthesized the first example of such a compound, bis(perchlorocatecholato)silane (pictured top). The team chlorinated catechol with H2O2/HCl and combined the product with HSiCl3 in acetonitrile to give an adduct of the desired super acid with two equivalents of acetonitrile in good yield. This procedure is scalable.
The product is able to bind halide ions from a range of reagents, and can abstract fluoride ions from SbF6–. The researchers were also able to synthesize acetonitrile-free bis(perchlorocatecholato)silane. According to them, the compound could have applications in bond activation and other fields of research.
- Bis(perchlorocatecholato)silane-A Neutral Silicon Lewis Super Acid,
Rezisha Maskey, Marcel Schädler, Claudia Legler, Lutz Greb,
Angew. Chem. Int. Ed. 2018.
https://doi.org/10.1002/anie.201712155