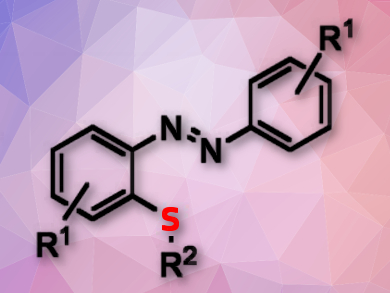
Azobenzenes with thiol substituents are common in natural products, functional materials, and bioactive compounds. There are some selective reactions for the thiolation of indolines or ketazines and extending these reactions to azobenzenes would be useful.
Xiangge Zhou, Sichuan University, Chengdu, China, and colleagues have developed a direct Rh-catalyzed ortho-C–H activation and functionalization of azobenzenes with diaryl disulfides. The team used [Cp*RhCl2]2 (Cp* = pentamethylcyclopentadienyl) as the catalyst, AgBF4 as an oxidant, and dichloroethane (DCE) as the solvent. Using this approach, a broad range of azobenzenes were thiolated with high regioselectivity to form mono-ortho-substituted products in moderate to excellent yields.
The reaction tolerates a variety of functional groups and the catalyst system could also be used to synthesize benzothiophenes and selenylated compounds.
- Rh(III)-Catalyzed Thiolation of Azobenzenes,
Xiangge Zhou, Jun Yang, Bowen Deng, Xiaofei Guo, Zhengkai Li, Haifeng Xiang,
Asian J. Org. Chem. 2017.
https://doi.org/10.1002/ajoc.201700641