Self-assembly is a set of natural phenomena that is responsible for creating order from disordered components. A few different mechanistic models have been suggested to explain these processes. Within chemistry, self-assembly has been intensively studied in supramolecular systems where rapid assembly-disassembly and vibration-driven reorganization models were suggested. These mechanisms cannot, however, explain the self-assembly of molecular species, as covalent bonds are often too strong for rapid reversible reactions.
Ronny Neumann, Weizmann Institute of Science, Israel, and colleagues have developed a mechanistic model to explain such chemical reactions. According to the model, reactant molecules first organize into supramolecular structures that then react internally. Thus, the organization and bonding steps that are required for such a reaction to occur are separated.
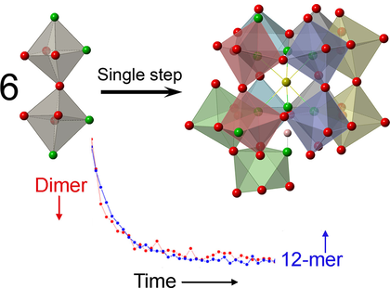
The developed model was demonstrated by NMR and MS methods on the self-assembly of an 18-tungsten polyoxometalate, from a reactant containing one tungsten atom. The same model may, in the future, contribute towards the engineering of self-assembly reactions for specific products as well as the development of multiple-electron transfer reactions.
- Self-Assembly through Noncovalent Preorganization of Reactants: Explaining the Formation of a Polyfluoroxometalate,
Roy E. Schreiber, Liat Avram, Ronny Neumann,
Chem. Europ. J. 2017.
https://doi.org/10.1002/chem.201704287