Organic chemists traditionally build molecular complexity by manipulating reactive carbon–heteroatom (C–X) and acidic carbon–hydrogen bonds, while leaving non-acidic C–H and carbon–carbon bonds intact. Unreactive C–H bonds are typically functionalized into reactive C–X bonds, however, the usefulness of such methods is limited by a lack of selectivity.
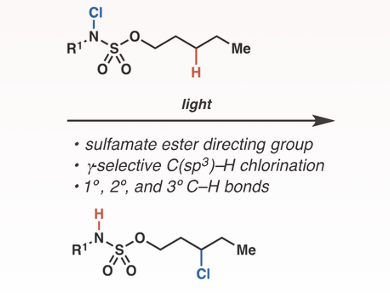
Jennifer L. Roizen and colleagues, Duke University, Durham, NC, USA, have developed a method by which unactivated aliphatic γ-C(sp3)–H bonds can be selectively functionalized. The team used blue light emitted by a light-emitting diode (LED) to homolyze the N–Cl bonds of N-chlorosulfamate esters. The resulting nitrogen-centered radical promotes intramolecular 1,6-hydrogen atom transfer, which selectively generates a γ-methylene carbon-centered radical. Subsequent γ-chlorination produces alkyl chloride.
A range of synthetically useful alkyl chlorides can be prepared with the method. Sulfamate esters are accessed from readily available alcohols, N-chlorination of N–H bonds of varying acidity is tolerated, and functionalization of C–H bonds at secondary, tertiary, and benzylic centers is possible.
- Sulfamate Esters Guide Selective Radical-Mediated Chlorination of Aliphatic C−H Bonds,
Melanie A. Short, J. Miles Blackburn, Jennifer L. Roizen,
Angew. Chem. Int. Ed. 2017.
https://doi.org/10.1002/anie.201710322