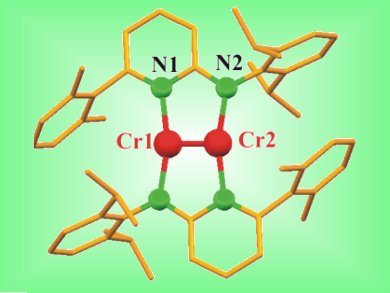
Bonds with very high bond orders are a fascinating subject of study and are often found in transient diatomic molecules, such as M2 (M = V, Nb, Cr, Mo), of which Cr2 is the best known.
Rhett Kempe and colleagues, University of Bayreuth, Germany, investigated the addition of alkynes and dienes to a quintuply bonded dichromium complex and observed cycloaddition reactions. Examination of the obtained structures indicates that reduction of the alkyne and diene ligands, oxidation of the chromium atoms, and a decrease in the Cr–Cr quintuple bond order occurred. These findings suggest that quintuple bonds might not be as enigmatic as initially thought. Indeed, they show similarities in reactivity to the simple and well-understood C–C double and triple bonds.

Image: (c) Wiley-VCH
- Cycloaddition Reactions of a Chromium-Chromium Quintuple Bond
A. Noor, E. S. Tamme, S. Qayyum, T. Bauer, R. Kempe,
Chem. Eur. J. 2011, 25.
DOI: 10.1002/chem.201100841