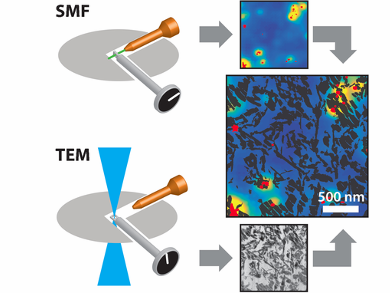
Petroleum refineries use fluid catalytic cracking (fcc) to convert high molecular weight hydrocarbons into valuable chemicals. Fcc catalysts — porous particles containing zeolite, clay, alumina, and binder materials — are integral to this process, but deactivate over time. Activity decline is correlated with changes to the structural features and composition of fcc catalysts. However, it is very challenging to characterize this relationship on the nanoscale.
Bert M. Weckhuysen, Hans C. Gerritsen, and colleagues, Utrecht University, The Netherlands, have developed a nanoscale imaging method, which integrates single-molecule fluorescence and transmission electron microscopies (SMF-TEM), and have used this to overlay high-resolution structural and reactivity data for a single fcc catalyst particle. The team prepared 100 nm thin slices of fcc catalyst particles and applied these as catalysts in thiophene oligomerization under high vacuum conditions.
SMF microscopy detected fluorescent species at the active acid sites, whereas high-resolution TEM was able to visualize physical features 100 times smaller than a virus. High reactivity in a thiophene oligomerization probe reaction correlated well with TEM-derived zeolite locations, while matrix components, such as clay and amorphous binder material, were found not to display activity. Differences in fluorescence intensity were also observed within and between distinct zeolite aggregate domains, indicating that not all zeolite domains are equally active. The team suggest that this method could be extended to other functional materials.
- Integrated Transmission Electron and Single-Molecule Fluorescence Microscopy Correlates Reactivity with Ultrastructure in a Single Catalyst Particle,
Frank C. Hendriks, Sajjad Mohammadian, Zoran Ristanović, Sam Kalirai, Florian Meirer, Eelco T. C. Vogt, Pieter C. A. Bruijnincx, Hans C. Gerritsen, Bert M. Weckhuysen,
Angew. Chem. Int. Ed. 2017.
https://doi.org/10.1002/anie.201709723