Peptidomimetics are small protein-like chains designed to mimic peptides. They are used in the development of drugs. In the search for peptidomimetics with improved properties such as potency, bioavailability, selectivity, and stability against proteolysis, it is a major strategy to incorporate conformationally restricted α-amino acids into biologically active peptides.
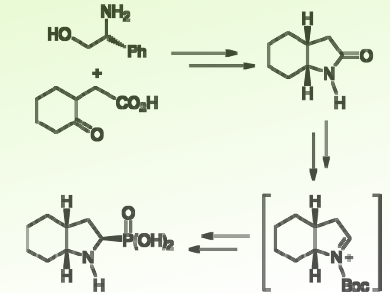
One of the most intensively investigated conformationally constrained α-amino acids is octahydroindole-2-carboxylic acid (Oic), a proline derivative that incorporates a fused cyclohexane ring into the pyrrolidine framework. Several synthetic methods for its stereoselective preparation are available. However, the stereoselective synthesis of its phosphonic analogs (OicP) has not yet been described, despite the great importance that these compounds could show in medicinal and organic synthesis.
José Luis Viveros-Ceballos, Universidad Autónoma del Estado de Morelos, Mexico, and colleagues have reported the first stereoselective synthesis of (2R,3aR,7aR)- and (2S,3aS,7aS)-octahydroindole-2-phosphonic acid (OicP derivatives). It involves two steps: First, the formation of two contiguous chiral centers through a dynamic kinetic resolution of racemic γ-keto acid with (R)- and (S)-phenylglycinol via Meyers’ bicyclic lactams to give the enantiomerically pure cis-fused bicyclic (3aR,7aR)- and (3aS,7aS)-pyrrolidine-2-ones. Second, the highly diastereoselective addition of trimethyl phosphite to N-acyliminium ion intermediates.
- First and Highly Stereoselective Synthesis of Both Enantiomers of Octahydroindole-2-phosphonic Acid (OicP),
José Luis Viveros-Ceballos, Erick Iván Martínez-Toto, César Eustaquio-Armenta, Carlos Cativiela, Mario Ordóñez,
Eur. J. Org. Chem. 2017.
https://doi.org/10.1002/ejoc.201701330