Studies of small molecules used as therapeutic and diagnostic agents have provided a relatively clear picture of dose dependencies in the pharmacokinetics and clearance of these molecules from the body. However, the properties of nanoscopic particles used as such agents are rather less well understood from this perspective.
Jie Zheng, The University of Texas at Dallas, Richardson, USA, and colleagues have worked on improving this understanding. The team has focused on dose dependency, biocompatibility, and clearance of gold nanoparticles by the mammalian kidney in animal models, including mice and non-human primates.
Paradoxical Pharma
The researchers explain how many small molecules used in medicine have strongly dose-dependent pharmacokinetics and clearance in animal models and in patients. For example, they say, increasing the dose of the broad-spectrum penicillin-type antibiotic mezlocillin from 20 mg/kg body weight to 200 mg/kg leads to a halving of the plasma clearance rate. This is caused by a saturation of both the biliary (via bile) and renal (via the kidneys) excretion pathways.
By contrast, clearance of the relatively new carbapenem antibiotic MK-826 from blood plasma takes place five times more quickly when a similar increase in dose is made from 10 to 180 mg/kg. This boost is due to concentration-dependent protein binding. Other clearance effects might arise because of conjugates attached to a small molecule drug and their impact on prolonging time in blood circulation.
For engineered nanoparticles, there is little available information about this phenomenon. However, it is known that many types of nanoparticles can accumulate to severe levels in the reticuloendothelial system (cells throughout the body that are part of the immune system). This limits their translation from the laboratory to the clinic. Thankfully, those working with nanoparticles are aware of this drawback and have spent several years developing novel nanoparticles that can be cleared from the body by the kidneys as if the nanoparticles are themselves simply small molecules. Quantum dots and silica nanoparticles, for instance, are quickly cleared. There have been numerous systematic investigations of the renal processing of such nanoparticles following injection. The researchers state, “Pharmacokinetics and clearance become extremely critical to both fundamental understanding of their in vivo transport and future clinical translation.”
Nano Boost
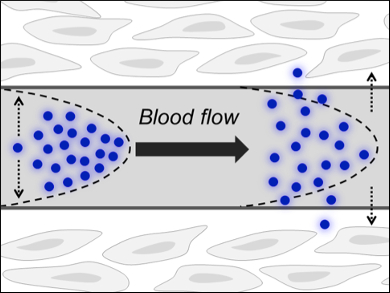
Gold nanoparticles are among the most well-studied nanoparticles, with a range of preparative techniques, detailed profiling, and applications. Gold nanoparticles that can be cleared from the body through the kidneys exist, however, clearance rates are typically low. For example, half the injected dose of 2 nm glutathione-coated gold nanoparticles (AuNPs) will still be present in the blood twenty-four hours after administration.
The problem is that blood is comparatively thick and the nanoparticles—as relatively bulky entities—move only slowly in the laminar blood flow (pictured). Their flow rate is dependent on the density and, given gold’s high atomic mass, AuNPs become subject to this slow way of excretion. Of course, a low clearance rate can be exploited in passive tumor targeting, for instance, in which a high clearance rate would mean only a tiny fraction of an injected dose of medical AuNPs would reach the disease site.
Advanced Understanding
The team explains that any boost to the use of nanoparticles in medicine will require a better understanding of such phenomena. The team has looked at near-infrared (NIR)-emitting 2.5 nm glutathione-coated AuNPs for their potential in imaging cancerous tumors and the kidney itself. They administered seven different doses ranging from 0.15 to 1059 mg/kg. Even at the maximum dose in mice, the team saw no trauma to the kidney and was able to quantify the pharmacokinetics of these nanoparticles clearly. Moreover, the animals respond positively to the higher dose in that clearance levels are maintained. Thus, the nanoparticles do not accumulate and toxic effects do not become apparent.
“These results significantly advance our fundamental understanding of the in vivo transport of NPs at different doses and lay down a foundation for optimizing the injected dose to maximize their potential in the future clinical practices,” the team reports. “Hopefully, in the near future, we can evaluate the renal clearable gold nanoparticles in clinical trials once we completely understand and can precisely control their targeting and clearance,” Zheng told ChemViews Magazine.
- Dose Dependencies and Biocompatibility of Renal Clearable Gold Nanoparticles,
Jie Zheng, Jing Xu, Mengxiao Yu, Chuanqi Peng, Phoebe Carter, Jia Tian, Xuhui Ning, Qinhan Zhou, Qiu Tu, Greg Zhang, Anthony Dao, Xingya Jiang, Payal Kapur, Jer-Tsong Hsieh, Xudong Zhao, Pengyu Liu,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201710584