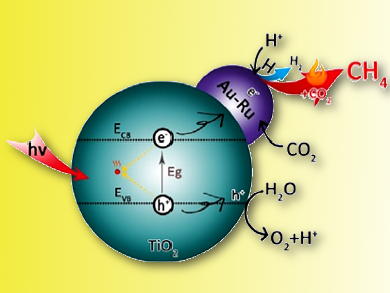
Photocatalytic reduction of CO2 into renewable hydrocarbon solar fuels is considered as a promising strategy to simultaneously address global energy and environmental issues.
Jingdong Lin, Xiamen University, China, and colleagues have developed an easy and highly efficient strategy for alkane production by using CO2 and solar energy. They directly coupled photocatalytic water splitting and thermocatalytic hydrogenation of CO2 on a bifunctional Au-Ru/TiO2 catalyst with active sites for both photocatalytic water splitting and thermocatalytic hydrogenation of CO2 to convert CO2–H2O into fuels.
The direct coupling of thermo- and photocatalysis over Au−Ru/TiO2 leads to 15 times higher activity (T = 358 K; ca. 99 % CH4 selectivity) in the conversion of CO2–H2O into fuels than purely photocatalytic water splitting. This is attributed to the promoting effect of thermocatalytic hydrogenation of CO2 with highly active hydrogen atoms generated in situ by photocatalytic water splitting, which can greatly reduce the recombination rate of photogenerated electrons and holes.
According to the researchers, the direct coupling of photocatalysis with thermocatalysis may be further extended to other hydrogenation reactions, such as ammonia synthesis or COx to methanol, with hydrogen derived in situ by solar energy.
- Direct Coupling of Thermo- and Photocatalysis for Conversion of CO2–H2O into Fuels,
Li Zhang, Guoguo Kong, Yaping Meng, Jinshu Tian, Lijie Zhang, Prof. Shaolong Wan, Prof. Jingdong Lin and Prof. Yong Wang,
ChemSusChem 2017.
https://doi.org/10.1002/cssc.201701472