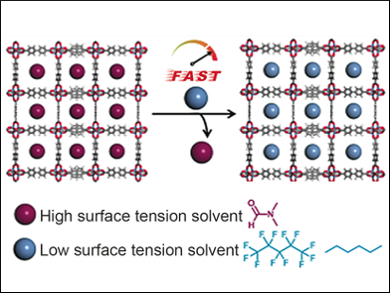
Metal–organic frameworks (MOFs) could be used, e.g., to capture pollutants or as the part of fuel cells that store hydrogen gas. MOFs are often made in solvents with high boiling points. They then need to be treated with solvents with low boiling points to remove the guest molecules, a process called activation. This process can take several days. Furthermore, this process uses solvents with high surface tension, which causes structures in fragile MOFs to collapse. To solve this problem, chemists have used supercritical carbon dioxide processing, but that requires specialized equipment.
Adam J. Matzger, University of Michigan, Ann Arbor, USA, and colleagues have studied the critical steps of MOF activation. The team used 1H NMR spectroscopy to observe the solvent exchange process between dimethylformamide (DMF) and CH2Cl2, using MOF-5 derivatives as model systems. The researchers found that solvent exchange kinetics are actually extremely fast. Based on these results, minutes rather than days could be the appropriate duration for solvent exchange in many MOFs.
In addition, solvents with ultra-low surface tensions can successfully activate MOFs that are usually challenging to prepare. They lower the capillary forces that could lead to structural collapse. Two delicate, highly porous MOFs, UMCM-9 and FJI-1, can achieve maximum surface area using solvents such as n-hexane or perfluoropentane. These solvents can activate MOFs in a matter of minutes, which is promising for the production of industrially important adsorbents.
- Rapid Guest Exchange and Ultra-Low Surface Tension Solvents Optimize Metal-Organic Framework Activation,
Jialiu Ma, Andre P. Kalenak, Antek G. Wong-Foy, Adam J. Matzger,
Angew. Chem. Int. Ed. 2017, 56, 14618–14621.
DOI: 10.1002/anie.201709187