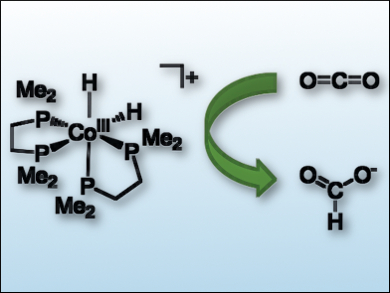
Two areas at the forefront of catalysis are rational design and the replacement of precious metals with first-row transition metals. In the field of CO2 hydrogenation, there are few examples of first-row transition metal hydrogenation catalysts that operate in water.
Eric S. Wiedner and colleagues, Pacific Northwest National Laboratory, Richland, WA, USA, have demonstrated that the choice of solvent can have a large influence on catalysis. Using thermodynamic principles as a predictive tool, the team found that a cobalt catalyst (pictured) for CO2 hydrogenation operates by different mechanisms in organic solvents and in water. By moving to an aqueous solution, a dihydride intermediate reacted directly with CO2 without first being deprotonated by an organic superbase.
The studied cobalt catalyst is more efficient than other first-row metal catalysts for the hydrogenation of CO2 in aqueous solution. These results demonstrate that rational design of catalysts can be achieved by understanding and controlling the thermodynamics of each reaction step during catalysis. Features beyond the active site, such as the solvent, are important for the design of new catalyst systems.
- Changing the Mechanism for CO2 Hydrogenation Using Solvent-Dependent Thermodynamics,
Samantha A. Burgess, Aaron M. Appel, John C. Linehan, Eric S. Wiedner,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201709319